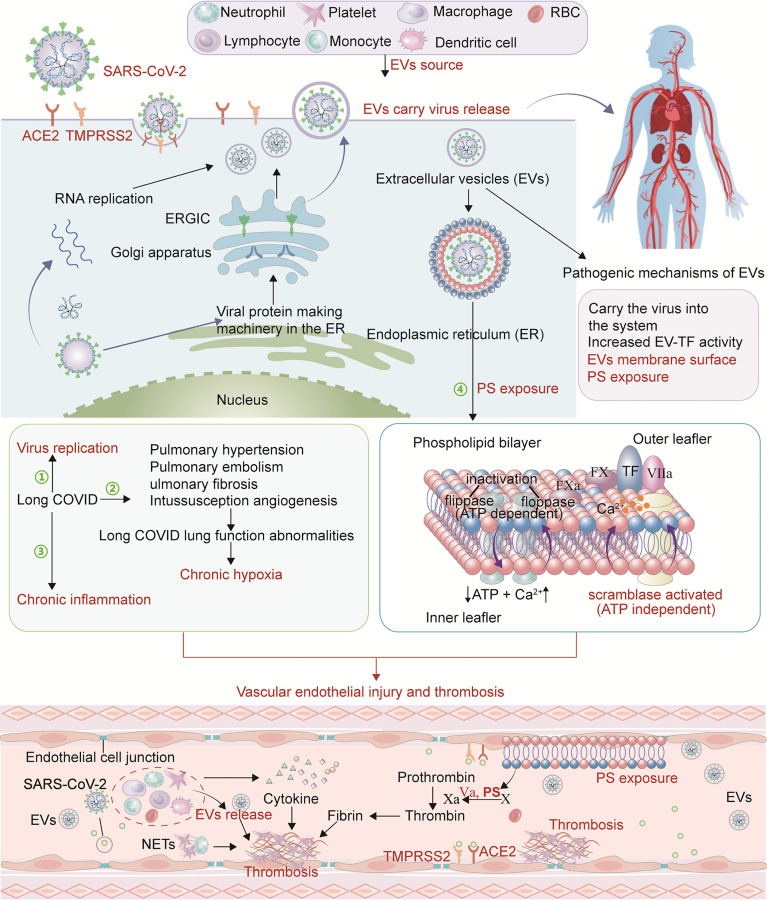
Figure 1.
Pathophysiological mechanism of long COVID thrombosis. SARS-CoV-2 enters cells through ACE2 and TMPRSS2 receptors and conducts RNA and protein synthesis and replication. SARS-CoV-2 buds in the ERGIC compartment or Golgi apparatus and exits the cell via a biosynthetic secretory pathway. In long-COVID, SARS-CoV-2 may hide in these EVs and re-attack various tissues and organs through the circulatory system. In addition, PS exposure on EVs creates a catalytic surface for clotting factors to facilitate the conversion of prothrombin to thrombin. After cell activation and injury, ATP production is reduced and consumption increases. With the resulting increase in intracellular Ca2+, two ATP-dependent transposases (flippase and floppase) are blocked, and ATP-independent scramblases are activated. This leads to the exposure of PS in the outer cell membrane, accompanied by the shedding of microparticles (MPs). PS promotes the decryption of tissue factor (TF) to form TF-FVIIa complex and provides binding sites for procoagulant complexes (endogenous and exogenous fXase and prothrombinase) leading to the generation of thrombin. Pulmonary hypertension, pulmonary embolism and pulmonary fibrosis are common in long COVID resulting in impaired lung function. With the change of lung function, chronic hypoxia inevitably occurs. Hypoxia-induced inflammation may further exacerbate capillary dysfunction and promote thrombosis. Due to SARS-CoV-2 persistence, chronic inflammation in long COVID may be a mechanism that stimulates ECs, platelets and other inflammatory cells, promotes the upregulation of procoagulant factors, and destroys the protective function of vascular endothelium, thereby causing abnormal coagulation.