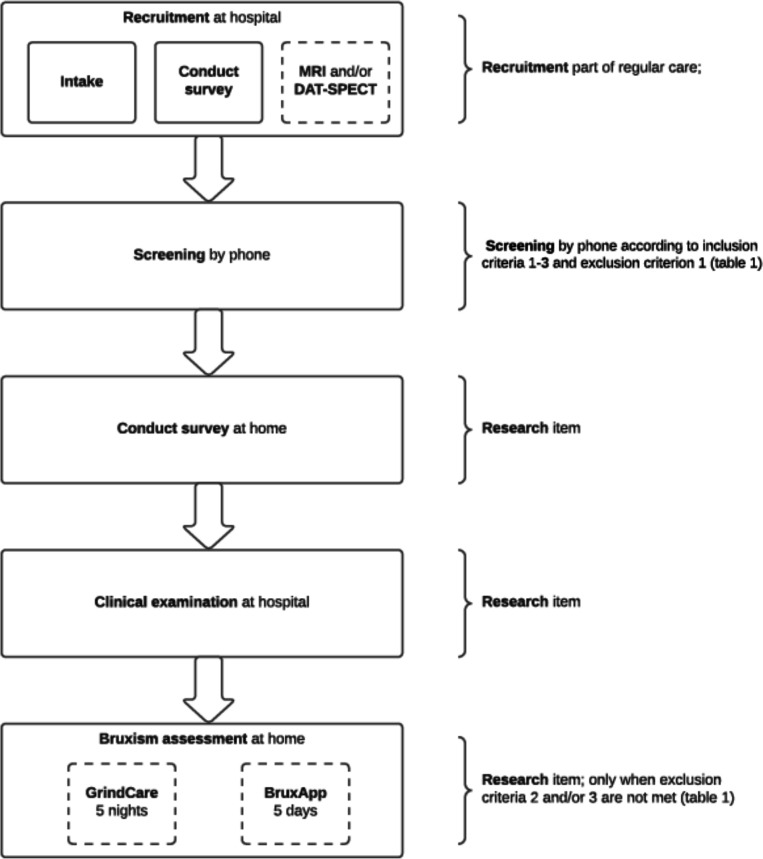
Figure 2.
Flow chart of the study in which a distinction was made between the attendance of participants at the hospital and the study components at the participant’s home. The intake and first survey is part of the regular care at the hospital, and only followed by an additional MRI and/or dopamine transporter single photon emission CT (DAT-SPECT) scan when indicated (dashed line). When patients are eligible and consent to participate (screening by phone), the additional data are collected after the intake. First, a questionnaire is filled in by the participants. After that, the participant is invited for the clinical examination. When questionnaires/screenings that are part of the regular care were filled in ≥1 year ago, participants will be asked to repeat this procedure simultaneously with the additional questionnaire and/or clinical examination. Finally, participants will sleep for five complete registration nights with the GrindCare for the assessment of sleep bruxism (when exclusion criterion two was not met) and use the BruxApp for five complete registration days for the assessment of awake bruxism (when exclusion criterion 3 was not met).