Abstract
Background
A vaccine (HB-101) consisting of 2 nonreplicating lymphocytic choriomeningitis virus (LCMV) vectors expressing the human cytomegalovirus antigens glycoprotein B (gB) and the 65-kD phosphoprotein (pp65), respectively, is in development to prevent cytomegalovirus infection.
Methods
HB-101 was tested in cytomegalovirus-naive, healthy adults in a randomized, double-blind, placebo-controlled, dose-escalation Phase I trial. Fifty-four subjects received low, medium, or high dose of HB-101 or placebo by intramuscular administration at Month 0, 1, and 3. Safety and immunogenicity were the respective primary and secondary endpoints. Subjects were followed for 12 months after the initial immunization.
Results
Vaccination was associated with transient mild to moderate adverse events. HB-101 administration induced dose-dependent gB- and pp65-specific cellular responses, dominated by pp65-specific CD8 T cells, a high fraction of which were polyfunctional. Two administrations were sufficient to elicit dose-dependent gB-binding and cytomegalovirus-neutralizing antibodies (Abs). Cytomegalovirus-specific immune responses were boosted after each administration. Only 1 of 42 vaccine recipients mounted a transient LCMV vector-neutralizing Ab response.
Conclusions
HB-101 was well tolerated and induced cytomegalovirus-specific polyfunctional CD8 T-cell and neutralizing Ab responses in the majority of subjects. Lack of vector-neutralizing Ab responses should facilitate booster vaccinations. These results justify further clinical evaluation of this vaccine candidate.
Keywords: cytomegalovirus, healthy volunteers, Phase I, vaccine, viral vector
We described the first-in-man study of a replication-deficient arenavirus-based vector, HB-101, expressing cytomegalovirus gB and pp65. Consecutive administrations of HB-101 were well tolerated and elicited gB-binding and neutralizing antibodies as well as cytomegalovirus-specific CD8 T-cell responses in healthy volunteers.
Infection with human cytomegalovirus is common [1]. Although the majority of cases are asymptomatic, cytomegalovirus infection can cause substantial morbidity and mortality in congenitally infected infants and immune-compromised adults, especially transplant recipients [2, 3]. Current treatment is limited to antiviral therapy, whereas a prophylactic vaccine remains unavailable for clinical use. Several vaccine candidates are in early-stage clinical development [4, 5].
The cytomegalovirus fusion protein, glycoprotein B (gB), is essential for viral entry and infection [6]. Antibodies specific for gB can interfere with entry of cytomegalovirus into all cell types evaluated [7, 8]. Monovalent, adjuvanted gB subunit protein vaccines have been extensively tested in clinical trials and elicited gB-binding and cytomegalovirus-neutralizing Abs (nAbs) with partial protection against cytomegalovirus infection [9–17]. The 65-kD phosphoprotein (pp65) is a key target of cellular immunity to cytomegalovirus [18]. A bivalent plasmid deoxyribonucleic acid vaccine candidate expressing gB and pp65 showed modest immunogenicity and effectiveness in humans [19–21].
We have reported positive efficacy outcomes in guinea pigs vaccinated with a novel replication-deficient lymphocytic choriomeningitis virus (rLCMV) vector expressing gB and pp65 orthologs of guinea pig cytomegalovirus [22]. Replication-deficient lymphocytic choriomeningitis virus vectors were chosen because they induce neutralizing antibodies (Abs) and CD8 T-cell responses against exogenous antigens in mice, guinea pigs, and monkeys [22–25]. Based on our preclinical data, we designed an rLCMV-based vaccine encoding the human cytomegalovirus antigens gB and pp65 [22] and evaluated this novel vaccine candidate, HB-101, in humans. The objectives of this first-in-human Phase I trial were to assess the safety and immunogenicity of 3 administrations of the candidate vaccine at ascending doses in healthy seronegative adult volunteers.
METHODS
Vaccine
The vaccine candidate HB-101 has previously been described [22]. The LCMV gene encoding the essential glycoprotein was replaced with genes encoding gB or pp65 protein. The gB-expressing rLCMV vector encodes a C-terminally truncated version of gB lacking the cytoplasmic domain derived from the cytomegalovirus strain Merlin, whereas the pp65-expressing rLCMV vector encodes an unmodified pp65 protein derived from the cytomegalovirus strain AD169 [22]. HB-101 is a mixture of equal quantities (focus-forming units [FFU]) of pp65- and gB-expressing vectors, formulated for injection and stored at < −65°C until use. The proprietary vaccine formulation buffer was used as placebo.
Subject Enrollment
The study protocol was approved by the Institutional Ethics Committee of the University Hospital Ghent and the Belgian Federal Agency for Medicines and Health Products. The clinicaltrials.gov number was NCT02798692. The randomized, double-blind, sequential, parallel cohorts, dose-escalation Phase I study was conducted at the Center for Vaccinology, Ghent, Belgium, in accordance with Good Clinical Practice. An independent data and safety monitoring board regularly reviewed the data. Written informed consent was obtained from all participating subjects.
Healthy adults aged 18–45 years who were cytomegalovirus seronegative by a Chemiluminescence Microparticle Immune Assay were eligible for the study. Exclusion criteria comprised pregnancy or unwillingness to practice birth control (women of child-bearing potential), serological signs of human immunodeficiency virus or hepatitis B and/or C virus infection, previous vaccination with an investigational cytomegalovirus vaccine, or any vaccination other than for seasonal influenza within 3 months before study entry, confirmed or suspected immunodeficiency or autoimmune disorders, and working as a childcare provider.
Randomization and Masking
Eighteen subjects were recruited into each of 3 sequential cohorts (low dose, medium dose, high dose). Each cohort was randomized at a 14:4 ratio between vaccine and placebo.
The study was double-blind. Both the investigator and the subject ignored the treatment arm (placebo/vaccine) to which the subject was allocated, up to the end of the study (Month 12). Syringes containing the test article (placebo or vaccine) to be administered were prepared by an unblinded pharmacist, independent of clinical staff, and were delivered to the clinical team for administration in a blinded manner. The immunogenicity data, potentially leading to the unblinding of the treatment groups, were not available during the course of the study to the investigator or any person involved in the clinical conduct of the study (including data cleaning), until the end of the active phase (Month 4 included).
Vaccine Administration
A vaccine (or placebo) volume of 0.5 mL was administered in the deltoid muscle of the nondominant arm at low (2.6 × 106 FFU), medium (2.6 × 107 FFU), or high (2.6 × 108 FFU) dose. Subjects received 3 intramuscular immunizations at Month 0, 1, and 3. The study was divided in 2 phases: an active treatment phase from Day 0 to Month 4 and a passive follow-up phase from Month 4 + 1 day to Month 12 post-first administration.
Monitoring of Safety
Subject diaries and scripted questions were used to collect local (administration site pain, induration, erythema, pruritus, and swelling) and general (malaise, fatigue, axillary body temperature, and generalized myalgia) adverse events (AEs) that occurred within 7 days after each administration. Unsolicited AEs were recorded through open-ended inquiries for 28 days after each administration. Intensities of AEs were graded as mild, moderate, severe, or potentially life-threatening and monitored throughout the active phase. Serious AEs (SAEs) were monitored throughout the study to Month 12. Blood (for hematology including complete blood count [CBC] and comprehensive metabolic panel [CMP]) and urine (for blood, proteins, and leucocytes) samples were collected on Day 0, 7, 28, 35, 84, 91, and Month 4.
Statistical Analysis
The sample size was calculated for an initial assessment of the safety and immunogenicity; however, the study was not powered for any statistical hypothesis testing. Exploratory inferential statistical tests were performed for immunogenicity results.
One subject in the medium dose group showed a strong increase in cytomegalovirus-specific responses between Month 4 and 6. Cytomegalovirus infection was confirmed by additional testing with immunoglobulin (Ig)M and IgG immunoblots [26, 27] and cytomegalovirus-specific quantitative real-time polymerase chain reaction using cytomegalovirus ELITe MGB kit (ELITech Group) by Tiziana Lazzarotto (University of Bologna, Italy). No symptoms were reported by the subject during this period. Results from this subject at Month 6 and 12 were excluded from the evaluation of cytomegalovirus-specific immune responses.
One subject from the low-dose group displayed an exceptionally high CD8 T-cell response (1.7%) against cytomegalovirus pp65 on Day 0 determined by intracellular cytokine staining (ICS), which was not observed in the same individual’s enzyme-linked immunospot (ELISPOT) analysis. Thus, this subject’s results were excluded from the ICS analysis.
Immunogenicity results were compared by Kruskal-Wallis tests between placebo and vaccine groups at Months 4 and 12, followed by Mann-Whitney’s tests for 2-by-2 comparisons, and by Friedman’s test followed by Wilcoxon’s test to compare between time points within the groups. P < .05 was considered statistically significant.
Polyfunctional ICS results were analyzed by merging the 3 vaccine cohorts and comparing the vaccine and the placebo groups using a linear mixed model and contrasted moderated t tests using P values adjusted for multiple testing [28, 29].
Additional materials and methods are provided in the Supplementary Methods. Gating strategy for flow-cytometry analysis is in Supplementary Figure S1.
RESULTS
Study Population
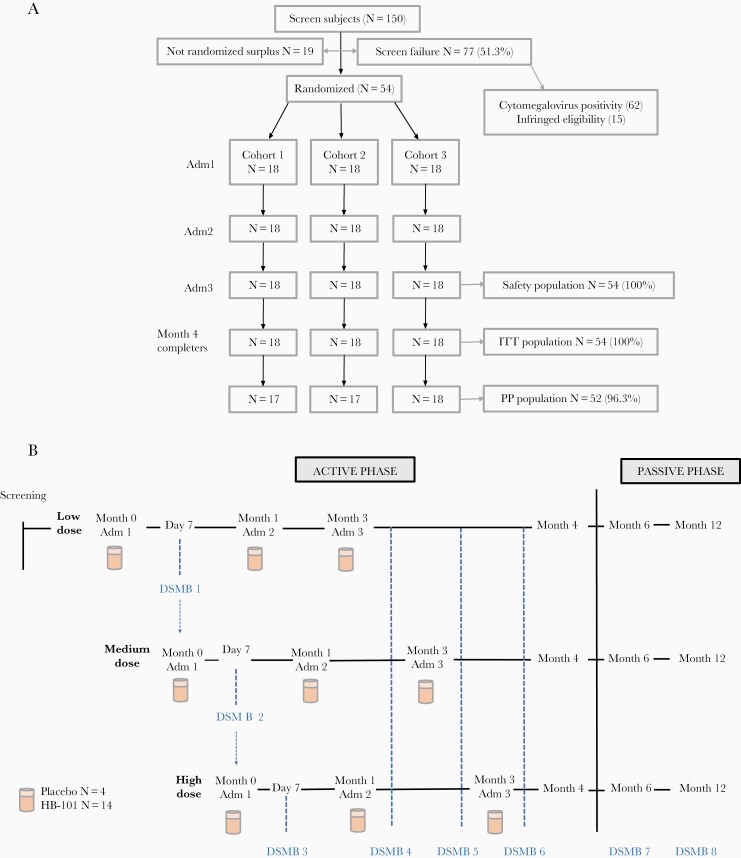
Of 150 subjects screened, 54 cytomegalovirus-negative subjects were enrolled and distributed into 3 cohorts of 18 subjects each (Figure 1A). Four randomly selected subjects in each cohort received placebo and 14 subjects received the vaccine. Enrolled subjects were 30.4 ± 7.5 (mean ± SD)-year-old Caucasians with a 4:5 male/female ratio (Supplementary Table S1). All subjects completed the active phase of the study and the safety analysis (Figure 1B). Two subjects were excluded from the per-protocol analysis because no peripheral blood mononuclear cells (PBMCs) could be collected on Day 14 from 1 subject and on Day 42 from the other subject. Consequently, these data are absent in the interferon (IFN)-γ ELISPOT and ICS assays. Fifty-three subjects completed the passive phase of the study (Figure 1A). One subject from Cohort 2 withdrew consent for personal reasons after Month 4 and was considered a dropout at Month 6 and 12.
Figure 1.
Clinical study design and organization. (A) Consort diagram. (B) Study flowchart. Adm, administration; DSMB, data and safety monitoring board; ITT, intent-to-treat; PP, per protocol.
Safety and Reactogenicity
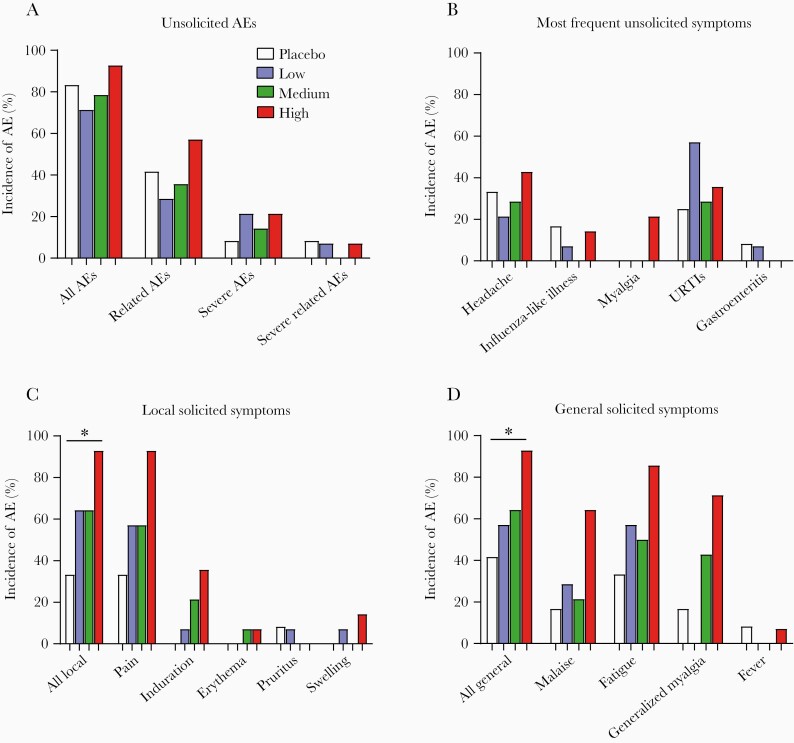
The primary objective was to assess the frequency and severity of AEs. Overall, the vaccine candidate was well tolerated at all dose levels, and no SAEs were reported. The percentage of subjects reporting unsolicited causally related AEs was similar among placebo and vaccine groups, whether analyzed as all AEs or divided into related, severe, and severe-related AEs (Figure 2A). Severe unsolicited causally related AEs were reported by 1 subject in the placebo group (headache), 1 subject in the low-dose cohort (gastroenteritis), and 1 subject in the high-dose cohort (headache) during the 28-day period after the 3 administrations. Headache and upper respiratory tract infection were the predominant unsolicited AEs (Figure 2B). No AE led to discontinuation of vaccine administration or dropout of subjects.
Figure 2.
Adverse events (AEs) reported in study subjects. (A–D) Percentage of subjects who reported unsolicited AEs (A), most frequent unsolicited symptoms (B), local solicited symptoms (C), and general solicited symptoms (D) after the 3 administrations in intent-to-treat population (placebo, N = 12; N = 14 per dose cohort). Statistical analysis was done on the percentage of subjects reporting any local or solicited symptoms at any time during the active phase of the study (all local in C, all general in D). For local solicited symptoms (C), the global difference between the 4 groups is significant (P = .020; Kruskal-Wallis test). The difference between placebo and high-dose group is significant (*, P = .003; Fisher’s exact test). The differences between other groups are not significant. For general solicited symptoms (D), the global difference between the 4 groups is borderline significant (P = .049; Kruskal-Wallis test). The difference between placebo and high-dose group is significant (*, P = .009; Fisher’s exact test). The differences between other groups are not significant. URTIs, upper respiratory tract infections.
Solicited local and general symptoms were of mild to moderate intensity (Supplementary Table S2), and 96.5% of reported symptoms resolved within 8 days postimmunization. Maximum duration of any symptom was 10 days. Pain at the injection site was the predominant solicited local AE (Figure 2C). Malaise, fatigue, and generalized myalgia were the most common solicited general symptoms (Figure 2D). Incidences of local and general solicited symptoms were significantly higher in the high-dose group compared with the placebo, whereas differences between other groups were not statistically significant (Figure 2C and D). The frequency of subjects reporting injection site pain increased with each subsequent administration; however, no cumulative effect was observed for general solicited AEs (Supplementary Figure S2). None of the subjects showed abnormal clinical chemistry results (CBC, CMP, and urine analysis) or vital signs.
Humoral Immune Responses
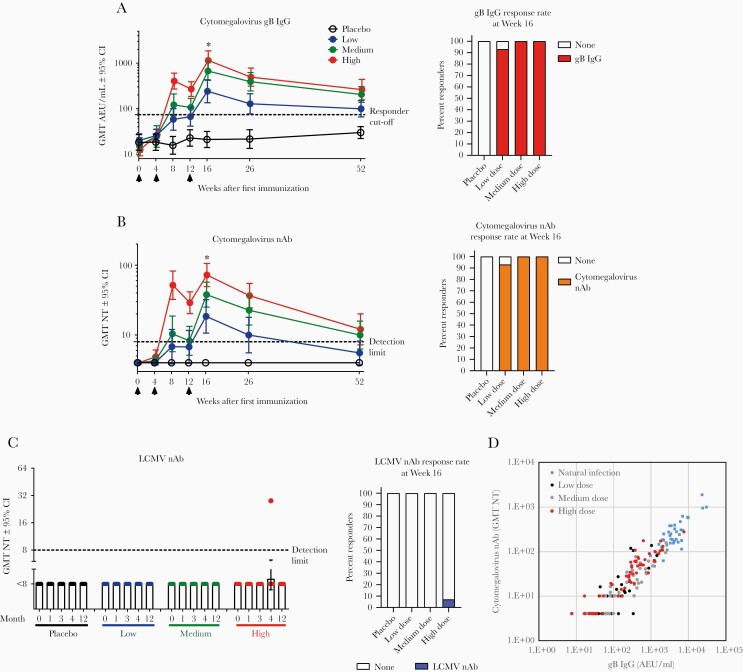
HB-101-induced humoral immune responses were evaluated. All individuals were seronegative on Day 0. In all dose groups, both gB-binding and cytomegalovirus nAbs were consistently detected after the second dose of HB-101 and continued to increase in magnitude after the third administration. Both gB-binding and cytomegalovirus nAbs peaked at approximately Week 16 (4 weeks after the third administration) and remained significantly above placebo up to Month 12. A dose-dependent response was found for both gB-binding and cytomegalovirus nAbs (Figure 3A and B).
Figure 3.
Humoral responses induced by HB-101 vaccination in healthy subjects. Antihuman cytomegalovirus glycoprotein B (gB) immunoglobulin (Ig)G (A), cytomegalovirus-neutralizing Abs (nAbs) (B), lymphocytic choriomeningitis virus (LCMV) nAbs (C), and correlation analysis of serum gB-binding and cytomegalovirus nAbs in vaccine recipients versus naturally infected individuals (D). Arrows indicate time of vaccine administration. Shown are geometric mean titers (GMTs) and 95% confidence intervals (CI). The GMTs of gB IgG and cytomegalovirus nAbs in naturally cytomegalovirus-infected donors were 3960 and 226, respectively (N = 40). For LCMV nAbs, 30 seronegative samples used as specificity controls had a titer <8 (detection limit), whereas 1 positive control donor with documented history of past LCMV infection had a titer of 88. Kruskal-Wallis’ test was used to compare the GMTs in the placebo and vaccine groups at Month 4 (*, P < .0001). Two-by-two comparisons indicated significant differences (P < .0001; Mann-Whitney’s test) between each vaccine dose group and placebo. Intragroup comparisons between Day 0 and all the other time points up to Month 4 revealed statistically significant differences (P < .0001; Friedman’s test) for the 3 vaccine doses in both gB-specific IgG titers and cytomegalovirus nAbs. Percentages of responders at Week 16 are illustrated on the right side of each response (A–C). Responders were those with measurements above the assay-specific cutoffs defining seropositivity (dashed line: [A] 74 arbitrary enzyme-linked immunosorbent assay units [AEU]/mL for anti-gB IgG, <8 for cytomegalovirus nAbs and LCMV nAbs). Samples with undetectable seroreactivity were arbitrarily assigned a value corresponding to 50% of the assay’s detection limit (A and B). Spearman’s test was used for correlation analysis, r = 0.92. NT, neutralizing titer.
All vaccinees (100%) in the high- and medium-dose groups mounted both gB-binding and cytomegalovirus nAbs after the third dose. In the low-dose group, the response rate was 93% (Figure 3B). In the high-dose group, 2 injections were sufficient to induce both gB-binding Ab and cytomegalovirus-neutralizing titers in 100% of subjects (Supplementary Figure S3A and B). At Month 12, all subjects receiving high dose retained gB-binding activity and 71% still had cytomegalovirus-neutralizing activity. In the medium-dose group, 2 injections elicited gB-binding Abs and cytomegalovirus-neutralizing titers in 71% and 57% subjects, respectively, and a third dose was required for all subjects to develop both responses (Supplementary Figure S3A and B).
No detectable rLCMV vector-neutralizing response was observed in 41 of 42 vaccine recipients. Vector-neutralizing activity was detected in 1 single subject in the high-dose group at Month 4 but was no longer detectable by Month 12 (Figure 3C and Supplementary Figure S3C).
Cytomegalovirus gB-specific IgG and cytomegalovirus nAb titers at Month 4 were highly correlated (Spearman’s test, r = 0.92) for all dose groups and followed a similar quantitative relationship as in serum samples of naturally cytomegalovirus-infected subjects (Figure 3D). These findings suggest that the gB immunogen in HB-101 mimicked the presentation of gB to the immune system in the context of natural cytomegalovirus infection.
Cellular Immune Responses
Interferon-γ Enzyme-Linked Immunospot
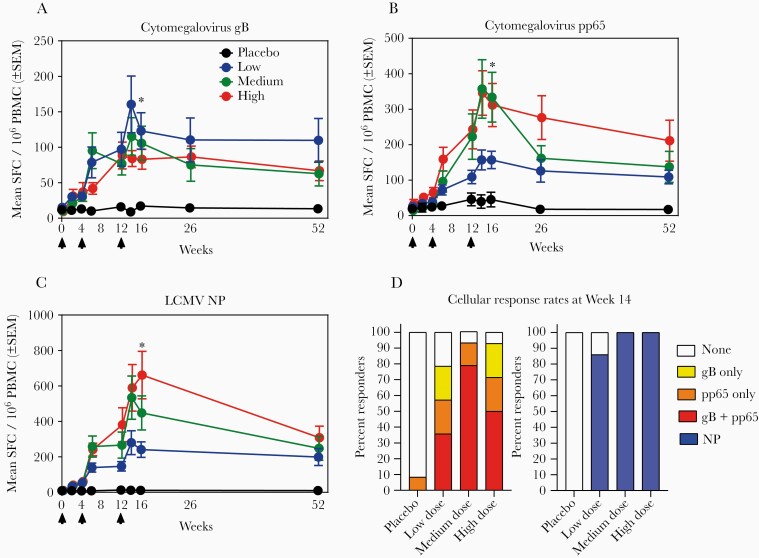
HB-101 induced significant cytomegalovirus gB- and pp65-specific IFN-γ-secreting cellular responses in all dose groups (P < .0001 compared with placebo at Month 4). Differences between the 3 dose groups were not significant (Figure 4A and B). A significant augmentation of all responses was observed after the second and third administration for both antigens in all dose groups, except for the gB responses in the high-dose cohort, the increase of which failed to reach statistical significance after the third dose. Up to Month 12, both gB- and pp65-specific IFN-γ-secreting PBMCs in the vaccine cohorts remained significantly higher than those in the placebo group (Figure 4A and B). HB-101 also induced significant vector-specific (LCMV nucleoprotein [NP]) IFN-γ-secreting cells in all dose groups (Figure 4C).
Figure 4.
Cellular responses induced by HB-101 vaccination in healthy subjects. Cytomegalovirus glycoprotein B (gB)-specific responses (A), cytomegalovirus pp65-specific responses (B), and lymphocytic choriomeningitis virus (LCMV) nucleoprotein (NP)-specific responses (C) were measured by enzyme-linked immunospot (ELISPOT). Arrows indicate time of vaccine administration. (A–C) Data shown are means ± standard error of the mean (SEM) of interferon (IFN)-γ-secreting cells per 106 peripheral blood mononuclear cells (PBMC). The mean response observed in reference samples from naturally cytomegalovirus-infected donors was 887 for gB (n = 7, range 42–2588) and 1733 for pp65 (n = 7, range 615–3742). Significant difference (*, P < .0001) were observed between placebo and vaccine groups at Month 4 (Kruskal-Wallis’s test) and between each vaccine dose group and placebo (Mann-Whitney’s test). P > .05 between each dose group (Mann-Whitney’s test). Intragroup comparisons revealed significant differences (P < .0001; Friedman’s test) between Day 0 and all the other time points up to Month 4 for the 3 vaccine doses in cytomegalovirus gB-, pp65-, and LCMV NP-specific IFN-γ-secreting cells. (D) Percentages of ELISPOT responders at Week 14. Responders to cytomegalovirus gB only, pp65 only, both gB and pp65, and NP were color coded. A responder was defined as subject with the mean spot-forming cells (SFC) >6-fold above individual baseline on Day 0.
After 3 administrations, 79% and 93% of low- and medium-/high-dose recipients, respectively, had responded to gB and/or pp65 (ELISPOT), whereas 86% (low dose) and 100% (medium and high dose) of subjects responded to LCMV NP (Figure 4D). These high response rates remained throughout the study up to Month 12 (Supplementary Figures S4A and S5A).
Flow-Cytometry Profiling of HB-101-Induced T-Cell Subsets
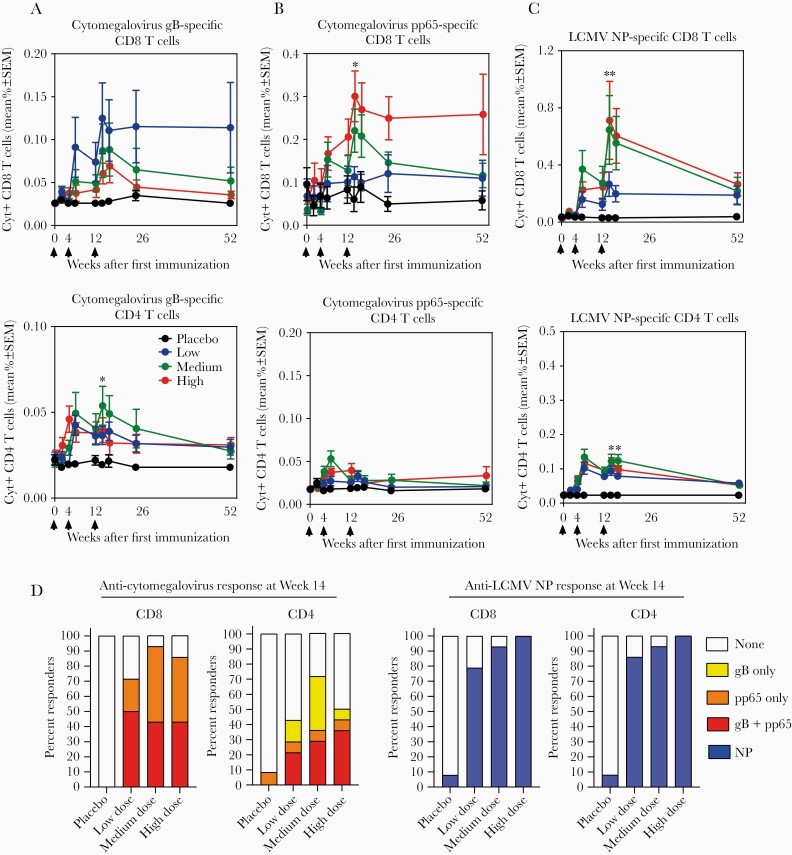
To assess the functionality and phenotype of T-cell subsets responding to HB-101, we performed intracellular cytokine assays for IFN-γ, interleukin (IL)-2, and tumor necrosis factor (TNF), in conjunction with CD107a surface detection as a surrogate of cytolytic granule release by CD8 T cells and CD40L expression by CD4 T cells, an activation marker, which also indicates helper function. Vaccine recipients mounted both functional CD8 and CD4 T cells specific for cytomegalovirus gB and pp65. These CD8 T cells showed minimal decline between Month 4 and 12 (Figure 5A and B). Significant LCMV NP-specific CD8 and CD4 T-cell responses to the vector backbone were detected (Figure 5C).
Figure 5.
Cytokine-positive CD4 and CD8 T-cell response induced by HB-101 vaccination in healthy subjects. Percentage of cytomegalovirus glycoprotein B (gB)-specific (A), pp65-specific (B), and lymphocytic choriomeningitis virus (LCMV) nucleoprotein (NP)-specific CD4 and CD8 T cells (C) secreting at least 1 of the cytokines, interferon-γ, interleukin-2, or tumor necrosis factor during the study period are illustrated. Arrows indicate time of vaccine administration. Data shown are means ± standard error of the mean (SEM). Significant differences (*, P < .05; **, P < .0001; Kruskal-Wallis’s test) between the placebo and vaccine groups at Month 4 for cytomegalovirus pp65 + CD8 T, cytomegalovirus gB + CD4 T, LCMV NP + CD4 and CD8 T cells, but not for cytomegalovirus pp65 + CD4 (P = .579) or gB + CD8 T cells (P = .065). (D) Percentages of CD8 and CD4 T-cell responders at Week 14. Responders to cytomegalovirus gB only, pp65 only, both gB and pp65, and NP are color coded. A responder was defined as a subject whose frequency of cytokine-secreting CD4 or CD8 T cells increased to >2-fold the individual’s baseline on Day 0.
Cytokine-positive CD8 T-cell responses specific for gB and/or pp65 were detected in 71%, 93%, and 86% of the low-, medium-, and high-dose recipients, respectively, after 3 administrations (Figure 5D). The majority of subjects in all dose groups also exhibited cytokine-positive CD8 T cells reactive to LCMV NP. CD4 T-cell responses were observed up to 71% and 100% of vaccinees for gB/pp65 and LCMV NP, respectively, depending on the vaccine dose (Figure 5D). Most of these responses were detected up to Month 12 (Supplementary Figures S4 and S5).
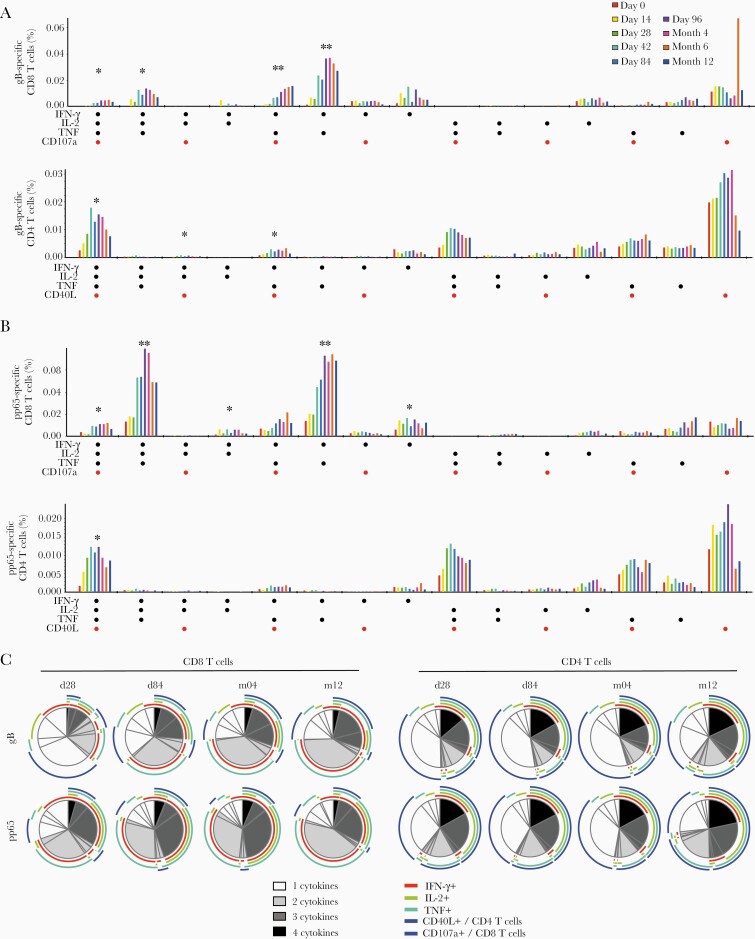
The polyfunctionality of HB-101-induced T-cell responses was investigated in an analysis of cytokine production (IFN-γ, IL-2, and TNF), CD107a (CD8 T cells), and CD40L expression (CD4 T cells). Several polyfunctional cytomegalovirus gB- and pp65-specific CD8 T subsets were detected. Most cells coexpressed IFN-γ and TNF, often in combination with IL-2 or CD107a (Figure 6A and B). For example, magnitudes of pp65-specific IFN-γ, TNF, and IL-2 coproducing CD8 T cells in vaccine recipients were significantly higher compared with placebo controls at Months 4 and 12 (Figure 6B). Analogously, a significant population of CD4 T cells expressing all 3 cytokines in combination with CD40L (IFN-γ +IL-2+TNF+CD40L+) was detected at Month 4, representing one of the most prominent populations amongst both the gB- and pp65-specific CD4 T cells (Figure 6A and B).
Figure 6.
Functional profiling of HB-101 vaccination-induced T cells. (A and B) Based on Boolean gating analysis, 15 populations representing all possible combinations of interferon (IFN)-γ, interleukin (IL)-2, tumor necrosis factor (TNF), and CD40L (CD4) or CD107a (CD8) were analyzed at the indicated time points. All vaccine recipients in the 3 cohorts were analyzed as 1 group, regardless of dose. Mean frequencies of CD8 and CD4 T cells specific for cytomegalovirus glycoprotein B (gB) (A) and pp65 (B) are displayed. *, Significant differences between placebo and vaccine groups at Month 4; **, significant differences between placebo and vaccine groups at Month 4 and 12. (C) Distribution of polyfunctional cytomegalovirus gB- and pp65-specific CD8 and CD4 T cells was analyzed using SPICE software. Mean proportions of cells producing 1, 2, 3, or 4 cytokines are shown at Day 28, Day 84, Month 4, and Month 12. Subpopulations with 4 functions are shown in black, with 3 functions in dark gray, bifunctional cells are shown in light gray and monofunctional cells are shown in white. Individual markers contributing to (multi)functionality are indicated by color-coded arcs.
The fraction of cytomegalovirus antigen-specific CD8 T cells displaying at least 2 cytokines/functions increased with each administration and exceeded 60% by Day 84, and those expressing at least 3 functions exceeded 25% by Day 84. This pattern remained largely unaltered throughout Month 12 (Figure 6C, left panel). Among CD4 T cells, 30%–40% of gB-reactive cells and 40%–50% of pp65-reactive cells expressed 3 or all 4 markers and a majority exhibited at least 2 functionalities (Figure 6C, right panel). This cytokine/functionality pattern of the CD4 response was largely established by Day 28 and stayed essentially unchanged thereafter. Similar patterns of polyfunctionality and distribution were found in vector backbone-reactive (LCMV NP-specific) CD4 and CD8 T cells (Supplementary Figure S6).
DISCUSSION
This is the first clinical study to test the safety and immunogenicity of replication-deficient LCMV as a vaccine delivery system, which was used here to deliver 2 cytomegalovirus antigens. The trial demonstrated that 3 consecutive administrations of HB-101 (1) were well tolerated up to a dose of 2.6 × 108 FFU, (2) elicited gB-binding and cytomegalovirus-nAbs, and (3) stimulated pp65- and gB-specific, polyfunctional CD8 T-cell responses in the majority of vaccine recipients and that the cytomegalovirus-nAb persisted for at least 6 months, whereas anti-gB Ab and cytomegalovirus-specific CD8 T-cell responses remained for at least 9 months. These results are consistent with earlier reports on humoral and cellular responses in mice, guinea pigs, and nonhuman primates [22–25] and provide a clinical proof of principle for the novel and versatile LCMV-based vaccine delivery platform.
The safety and reactogenicity profile of HB-101 in the tested population was comparable to that reported by vaccines based on other viral vectors [30–33]. Because immunogenicity and frequency of solicited AEs were both dose-dependent, selection of a dose for further clinical development requires a careful risk/benefit analysis. In light of the data presented here, notably with a view on response rates, further clinical development of HB-101 at either the high or medium dose seems justified.
The present study evidenced a virtually complete absence of detectable vector nAb responses. This unique feature of the LCMV vaccine delivery platform is apparently due to the glycan shield of the arenavirus envelope protein, the sole target of vector nABs [34, 35]. Absence of antivector Ab responses allowed all cytomegalovirus antigen-specific immune responses to be significantly boosted upon repeated vaccine readministration. A lack of vector nAb responses is particularly relevant to vaccines that require repeated administration.
Humoral and cellular immunity contribute independently to protection against cytomegalovirus [36]; therefore, it stands to reason that combining them would lead to an additive or synergistic effect on vaccine efficacy. In our study, vaccination with HB-101 triggered cytomegalovirus-specific humoral responses in up to 100% of recipients and functional cellular responses in up to 93% of recipients. The cytomegalovirus-specific cellular response was dominated by the pp65-specific CD8 T-cell response, which is likely biologically relevant. Although comparison of CD8 responses between different trials is hampered by the lack of standardized measurement of cellular responses, the magnitude of pp65-specific CD8 T cells induced by HB-101 was in the range where adoptive CD8 T-cell transfer was reported to effectively control drug-resistant cytomegalovirus infection in stem cell transplant recipients [37, 38]. Likewise, HB-101 induced gB-specific, cytomegalovirus nAbs comparable to gB/MF59, the most effective cytomegalovirus vaccines tested to date, which has demonstrated partial efficacy against cytomegalovirus-related disease in high-risk transplant recipients and in vertical transmission [11, 15, 16]. Recent findings also suggest that the efficacy of gB/MF59 could be attributed, at least in part, to nonneutralizing gB-specific Abs [13]. It is noted that Abs to the cytomegalovirus pentameric complex gH/gL/UL128/UL130/UL131 (PC) can be potently neutralizing and make up a substantial portion of the nAb response to natural cytomegalovirus infection [39, 40]. This renders also PC an attractive vaccine immunogen, although the benefit to vaccine recipients has not yet been proven in the clinic. As other vaccine candidates will evaluate the benefit of PC-specific Abs, further clinical testing of HB-101 will help decipher the benefit of a combined gB-specific Ab and cytotoxic T-cell response and inform about the contribution of nAbs, non-nAbs, and CD4 and CD8 T cells to protection against cytomegalovirus.
The cellular immune responses to the HB-101 vaccine candidate were polyfunctional. Simultaneous expression of multiple cytokines and certain phenotypic markers by either CD4 or CD8 T cells has been proposed to represent a correlate of vaccine-mediated protection against various infectious diseases [41, 42]. A prominent subset of cytomegalovirus-specific CD8 T cells produced IFN-γ and TNF often in conjunction with IL-2 and/or CD107a. Such polyfunctional cells have been found to correlate with protection against cytomegalovirus viremia in patients with lung transplantation [43]. The triple-positive (IFN-γ +IL-2+TNF+) and double-positive (IFN-γ +TNF+) CD8 T-cell populations in our study were more prominent than those reported in a previous Phase 1 trial of an alphavirus-based replicon vaccine targeting cytomegalovirus pp65, gB, and IE1 [32]. Concomitantly with these cytomegalovirus-specific T-cell responses, LCMV NP-specific, IFN-γ-secreting T cells were induced by HB-101. The presence of these vector-specific T cells did not prevent boosting of cytomegalovirus gB- or pp65-specific T cells by HB-101. Whether and to which extent cytomegalovirus-specific and LCMV-specific T-cell responses may compete remains to be investigated.
One subject receiving the medium vaccine dose showed unusually strong immune response to cytomegalovirus, and an asymptomatic cytomegalovirus infection between Month 4 and 6 was confirmed. Given that the subject had no apparent clinical outcome, and that cytomegalovirus infections in healthy adults is very common and typically subclinical [1–3], the impact of HB-101 vaccination on the course of cytomegalovirus infection in this individual cannot be interpreted conclusively.
Limitations of our study consist in the dose range of HB-101 that was tested. Cytomegalovirus nAb titers at the time of peak responses increased significantly in a dose-dependent manner, and it is possible that a dose higher than 2.6 × 108 FFU may elicit even higher responses. Furthermore, cytomegalovirus nAb titers gradually dropped, suggesting that additional booster doses beyond 12 months may be needed to maintain protective immunity. Alternatively, the immunization schedule was arbitrarily chosen, and it is possible that a different schedule may result in responses of higher magnitude and/or duration. Finally, the subjects in the current study were all seronegative at the time of study initiation. Augmenting immune responses in latently infected individuals also represents an important goal of vaccination, notably in the context of solid organ transplantation. To fully characterize the immunogenicity of HB-101, seropositive subjects will be included in future studies.
CONCLUSIONS
In summary, the replication-deficient LCMV vector-based HB-101 vaccine candidate is well tolerated and induces humoral and cellular immune responses against cytomegalovirus in naive, healthy adult volunteers. The safety and immunogenicity data generated in this first-in-human study justify further clinical evaluation of HB-101. This bivalent vaccine engages both arms of the adaptive immune system and holds promise for prevention of clinically significant cytomegalovirus infection in transplant recipients and unborn children.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to the following individuals: Tiziana Lazzarotto (University of Bologna) for performing tests to confirm cytomegalovirus infection; Elizabeth Watson, Elisabeth Albrecht, and Xiaoping Qing (Hookipa) for regulatory, technical, and writing support; Fien De Boever, Cathy Maes, Annelies Aerssens, and the Center for Vaccinology (CEVAC) team for conducting the clinical trial; ECSOR for clinical trial management; Caprion Biosciences for immunogenicity assays; the members of the data and safety monitoring board for monitoring; and the volunteers who participated in the trial.
Financial support. This work was funded by Hookipa Pharma, Inc. and the Austrian Research Promotion Agency.
Potential conflicts of interest. M. S., K. K. O., and A. E. L. are employees and stock option holder of Hookipa Pharma, Inc. D. D. P. is a founder, shareholder, and consultant to Hookipa and serves as the company’s chief scientific officer. G. T., H. B., and T. P. M. are former Hookipa employees; T. P. M. owns stock options for Hookipa. G. L.-R. has received grant support from Hookipa through the University Hospital for the conduct of this study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part. 6th International Congenital CMV Conference/16th International CMV/betaherpesvirus Workshop, 2017, Noordwijkhout, the Netherlands; the 7th International Congenital CMV Conference/17th International CMV/betaherpesvirus Workshop, 2019, Birmingham, AL; World Vaccine Congress Europe, 2019, Barcelona, Spain; World Vaccine & Immunotherapy Congress West Coast, 2019, San Francisco, CA; World Vaccine Congress, 2018, Washington DC; European Congress of Clinical Microbiology and Infectious Diseases, 2018, Madrid, Spain; ID Week, 2018, San Francisco, CA; American Transplant Congress, 2019, Philadelphia, PA; Meeting on Advanced Immunization Technologies, 2019, Siena, Italy; Immunotherapies and Innovations for Infectious Diseases Congress, 2019, Lyon, France.
References
- 1. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010; 20:202–13. [DOI] [PubMed] [Google Scholar]
- 2. Fisher RA. Cytomegalovirus infection and disease in the new era of immunosuppression following solid organ transplantation. Transpl Infect Dis 2009; 11:195–202. [DOI] [PubMed] [Google Scholar]
- 3. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253–76. [DOI] [PubMed] [Google Scholar]
- 4. Schleiss MR, Permar SR, Plotkin SA. Progress toward development of a vaccine against congenital cytomegalovirus infection. Clin Vaccine Immunol 2017; 24:e00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diamond DJ, La Rosa C, Chiuppesi F, et al. . A fifty-year odyssey: prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev Vaccines 2018; 17:889–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isaacson MK, Compton T. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J Virol 2009; 83:3891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pötzsch S, Spindler N, Wiegers AK, et al. . B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog 2011; 7:e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandramouli S, Ciferri C, Nikitin PA, et al. . Structure of HCMV glycoprotein B in the postfusion conformation bound to a neutralizing human antibody. Nat Commun 2015; 6:8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baraniak I, Kropff B, Ambrose L, et al. . Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc Natl Acad Sci U S A 2018; 115:6273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernstein DI, Munoz FM, Callahan ST, et al. . Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine 2016; 34:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Griffiths PD, Stanton A, McCarrell E, et al. . Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011; 377:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell DK, Holmes SJ, Burke RL, Duliege AM, Adler SP. Immunogenicity of a recombinant human cytomegalovirus gB vaccine in seronegative toddlers. Pediatr Infect Dis J 2002; 21:133–8. [DOI] [PubMed] [Google Scholar]
- 13. Nelson CS, Huffman T, Jenks JA, et al. . HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc Natl Acad Sci U S A 2018; 115:6267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pass RF. Development and evidence for efficacy of CMV glycoprotein B vaccine with MF59 adjuvant. J Clin Virol 2009; 46(Suppl 4):S73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pass RF, Duliegè AM, Boppana S, et al. . A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J Infect Dis 1999; 180:970–5. [DOI] [PubMed] [Google Scholar]
- 16. Pass RF, Zhang C, Evans A, et al. . Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J Infect Dis 2011; 203:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wills MR, Carmichael AJ, Mynard K, et al. . The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol 1996; 70:7569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wloch MK, Smith LR, Boutsaboualoy S, et al. . Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis 2008; 197:1634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. . A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12:290–9. [DOI] [PubMed] [Google Scholar]
- 21. Vincenti F, Budde K, Merville P, et al. . A randomized, phase 2 study of ASP0113, a DNA-based vaccine, for the prevention of CMV in CMV-seronegative kidney transplant recipients receiving a kidney from a CMV-seropositive donor. Am J Transplant 2018; 18:2945–54. [DOI] [PubMed] [Google Scholar]
- 22. Schleiss MR, Berka U, Watson E, et al. . Additive protection against congenital cytomegalovirus conferred by combined glycoprotein B/pp65 vaccination using a lymphocytic choriomeningitis virus vector. Clin Vaccine Immunol 2017; 24:e00300-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Penaloza MacMaster P, Shields JL, Alayo QA, et al. . Development of novel replication-defective lymphocytic choriomeningitis virus vectors expressing SIV antigens. Vaccine 2017; 35:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flatz L, Hegazy AN, Bergthaler A, et al. . Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat Med 2010; 16:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flatz L, Cheng C, Wang L, et al. . Gene-based vaccination with a mismatched envelope protects against simian immunodeficiency virus infection in nonhuman primates. J Virol 2012; 86:7760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lazzarotto T, Ripalti A, Bergamini G, et al. . Development of a new cytomegalovirus (CMV) immunoglobulin M (IgM) immunoblot for detection of CMV-specific IgM. J Clin Microbiol 1998; 36:3337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chiereghin A, Pavia C, Gabrielli L, et al. . Clinical evaluation of the new Roche platform of serological and molecular cytomegalovirus-specific assays in the diagnosis and prognosis of congenital cytomegalovirus infection. J Virol Methods 2017; 248:250–4. [DOI] [PubMed] [Google Scholar]
- 28. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001; 125:279–84. [DOI] [PubMed] [Google Scholar]
- 29. Ritchie ME, Phipson B, Wu D, et al. . limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berencsi K, Gyulai Z, Gönczöl E, et al. . A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J Infect Dis 2001; 183:1171–9. [DOI] [PubMed] [Google Scholar]
- 31. Huttner A, Dayer JA, Yerly S, et al. ; VSV-Ebola Consortium . The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase ½ trial. Lancet Infect Dis 2015; 15:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernstein DI, Reap EA, Katen K, et al. . Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 2009; 28:484–93. [DOI] [PubMed] [Google Scholar]
- 33. La Rosa C, Longmate J, Martinez J, et al. . MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood 2017; 129:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sommerstein R, Flatz L, Remy MM, et al. . Arenavirus glycan shield promotes neutralizing antibody evasion and protracted infection. PLoS Pathog 2015; 11:e1005276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinschewer DD, Perez M, Jeetendra E, et al. . Kinetics of protective antibodies are determined by the viral surface antigen. J Clin Invest 2004; 114:988–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plotkin SA. Is there a formula for an effective CMV vaccine? J Clin Virol 2002; 25 (Suppl 2):S13–21. [DOI] [PubMed] [Google Scholar]
- 37. Feuchtinger T, Opherk K, Bethge WA, et al. . Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010; 116:4360–7. [DOI] [PubMed] [Google Scholar]
- 38. Neuenhahn M, Albrecht J, Odendahl M, et al. . Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 2017; 31:2161–71. [DOI] [PubMed] [Google Scholar]
- 39. Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol 2012; 86:7444–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macagno A, Bernasconi NL, Vanzetta F, et al. . Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 2010; 84:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thakur A, Pedersen LE, Jungersen G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine 2012; 30:4907–20. [DOI] [PubMed] [Google Scholar]
- 42. Janes HE, Cohen KW, Frahm N, et al. . Higher T-cell responses induced by DNA/rAd5 human immunodeficiency virus-1 preventive vaccine are associated with lower HIV-1 infection risk in an efficacy trial. J Infect Dis 2017; 215:1376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Snyder LD, Chan C, Kwon D, et al. . Polyfunctional T-cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med 2016; 193:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.