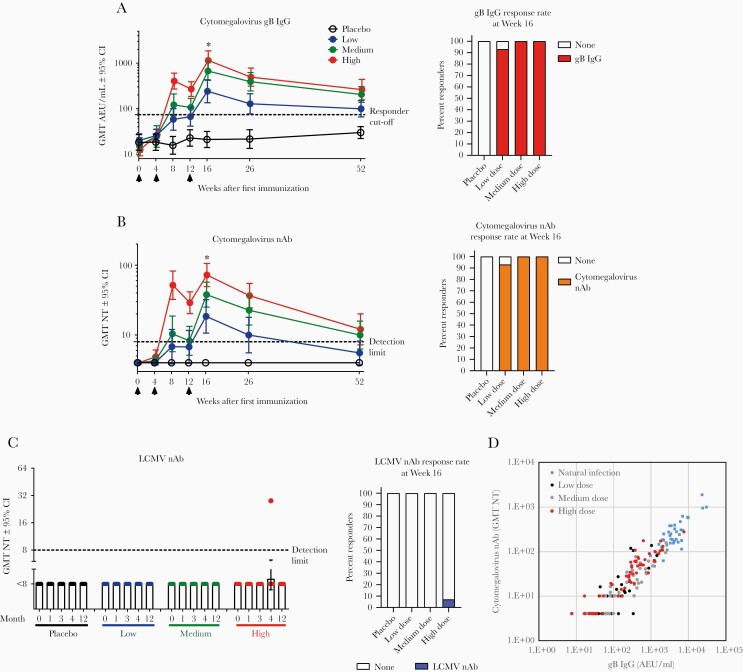
Figure 3.
Humoral responses induced by HB-101 vaccination in healthy subjects. Antihuman cytomegalovirus glycoprotein B (gB) immunoglobulin (Ig)G (A), cytomegalovirus-neutralizing Abs (nAbs) (B), lymphocytic choriomeningitis virus (LCMV) nAbs (C), and correlation analysis of serum gB-binding and cytomegalovirus nAbs in vaccine recipients versus naturally infected individuals (D). Arrows indicate time of vaccine administration. Shown are geometric mean titers (GMTs) and 95% confidence intervals (CI). The GMTs of gB IgG and cytomegalovirus nAbs in naturally cytomegalovirus-infected donors were 3960 and 226, respectively (N = 40). For LCMV nAbs, 30 seronegative samples used as specificity controls had a titer <8 (detection limit), whereas 1 positive control donor with documented history of past LCMV infection had a titer of 88. Kruskal-Wallis’ test was used to compare the GMTs in the placebo and vaccine groups at Month 4 (*, P < .0001). Two-by-two comparisons indicated significant differences (P < .0001; Mann-Whitney’s test) between each vaccine dose group and placebo. Intragroup comparisons between Day 0 and all the other time points up to Month 4 revealed statistically significant differences (P < .0001; Friedman’s test) for the 3 vaccine doses in both gB-specific IgG titers and cytomegalovirus nAbs. Percentages of responders at Week 16 are illustrated on the right side of each response (A–C). Responders were those with measurements above the assay-specific cutoffs defining seropositivity (dashed line: [A] 74 arbitrary enzyme-linked immunosorbent assay units [AEU]/mL for anti-gB IgG, <8 for cytomegalovirus nAbs and LCMV nAbs). Samples with undetectable seroreactivity were arbitrarily assigned a value corresponding to 50% of the assay’s detection limit (A and B). Spearman’s test was used for correlation analysis, r = 0.92. NT, neutralizing titer.