Abstract
Background
Protection against human respiratory syncytial virus (RSV) remains an unmet need potentially addressable by maternal immunization. This phase 1/2 study evaluated a bivalent prefusion F vaccine (RSVpreF) with antigens from RSV subgroups A and B.
Methods
Adults 18–49 years old (N = 618) were randomized to receive placebo or 60, 120, or 240 µg RSVpreF with or without Al(OH)3. Safety and immunogenicity were evaluated.
Results
RSVpreF recipients more frequently reported local reactions and systemic events than placebo recipients; these were mostly mild or moderate. No vaccine-related serious adverse events occurred through 12 months postvaccination. All RSVpreF formulations induced 1-month postvaccination virus-neutralizing titers higher than those associated with protection of high-risk infants by palivizumab, the only prophylactic currently available for RSV. Geometric mean fold rises (GMFRs) across RSVpreF doses/formulations were 10.6–16.9 for RSV A and 10.3–19.8 for RSV B at 1 month postvaccination, greater than those historically elicited by postfusion F vaccines. GMFRs were 3.9–5.2 and 3.7–5.1, respectively, at 12 months postvaccination.
Conclusions
RSVpreF formulations were safe, well tolerated, and induced robust neutralizing responses in adults. These findings support development of RSVpreF, which is being evaluated in a pivotal phase 3 study for maternal immunization.
Clinical Trials Registration
Keywords: F protein, immunogenicity, maternal vaccination, neutralizing responses, respiratory syncytial virus, safety, vaccine
Respiratory syncytial virus stabilized prefusion F subunit vaccine (RSVpreF) formulations were well tolerated and highly immunogenic in younger adults. These findings support further development of RSVpreF in a pivotal phase 3 study for maternal immunization.
Human respiratory syncytial virus (RSV) is the most common cause of severe acute lower respiratory tract illness in infants and an important cause of disease in older adults [1]. In the United States, most infants are infected during their first winter [2], with RSV-associated hospitalization rates of approximately 1%–2% and highest in the first months of life [3, 4].
Over 50 years of vaccine development efforts have not produced a licensed RSV vaccine [5]. The only available preventive is palivizumab (Synagis; MedImmune LLC), a neutralizing monoclonal antibody targeting RSV fusion glycoprotein (F) [6]. Palivizumab reduces RSV-related high-risk infant hospitalizations [7], supporting the ability of neutralizing antibodies to protect against severe disease; however, its practicality is limited by high cost [8] and up to 5 monthly injections each season [9]. Thus, an effective RSV vaccine remains a significant unmet medical need.
Pediatric RSV vaccine development has been impeded since the 1960s, when RSV-naive infants immunized with a formalin-inactivated vaccine candidate experienced enhanced disease upon subsequent natural RSV infection [5, 10–13]. This phenomenon was linked to the vaccine eliciting predominantly non-neutralizing antibodies [10] and a T helper 2 (TH2)-biased helper T-cell response [14]. In contrast, RSV infection elicits neutralizing antibodies and TH1 phenotype T-cell responses that are unassociated with disease enhancement upon RSV reexposure [14, 15]. Disease enhancement was not observed in individuals previously infected with RSV, regardless of subsequent immunization [11–13].
Pregnant women are universally RSV experienced [2], and maternally transferred virus-neutralizing antibodies have been linked to less-severe disease in infants [16]. Immunization during pregnancy could augment transplacental transfer of maternal antibodies to protect against disease [5, 17–20]. Maternal immunization also addresses the temporal challenge of protecting infants when most vulnerable to RSV and when direct protection is difficult to achieve with vaccination [21]. Moreover, immunization of pregnant women against tetanus, diphtheria, and pertussis (Tdap) and influenza is safe, effective, and well accepted [22–25].
Respiratory syncytial virus expresses 2 envelope glycoproteins, a heavily glycosylated attachment protein (G) [26, 27] and the type I fusion protein F [28]. Unlike the antigenically divergent RSV G (primary determinant of A and B subgroups) [29], RSV F carries multiple, relatively conserved neutralizing epitopes [30], making it a key vaccine antigen. Sequence variability in F between RSV subgroup A and B strains clusters in antigenic site φ, the target of very potent neutralizing antibodies [28, 31]. F on the virion exists in a metastable prefusion state, transitioning to a stable postfusion state while mediating virus entry into cells [32, 33]. Determination of prefusion F structure informed the engineering of a conformationally stable prefusion F immunogen at the US National Institutes of Health [28, 34]. In an exploratory clinical study, that stabilized immunogen elicited higher neutralizing titers than those obtained in any trial of an F-subunit vaccine candidate not stabilized in the prefusion conformation [35]. F-specific antibodies must bind their prefusion conformation to neutralize RSV (though some can also bind postfusion F) [36], partly explaining failures of postfusion F vaccine trials in adults [19, 37]. These data suggest a vaccine should include prefusion F immunogens from both subgroups A and B for maximal protection against circulating RSV strains.
This large phase 1/2 trial evaluated safety, tolerability, and immunogenicity of a novel bivalent prefusion F vaccine (RSVpreF) in adults. To initiate maternal, older adult, and high-risk adult RSV immunization programs, healthy adults 18–49 and 50–85 years old were recruited. This report summarizes results in adults 18–49 years of age; results in adults 50–85 years of age will be presented separately.
METHODS
Study Design
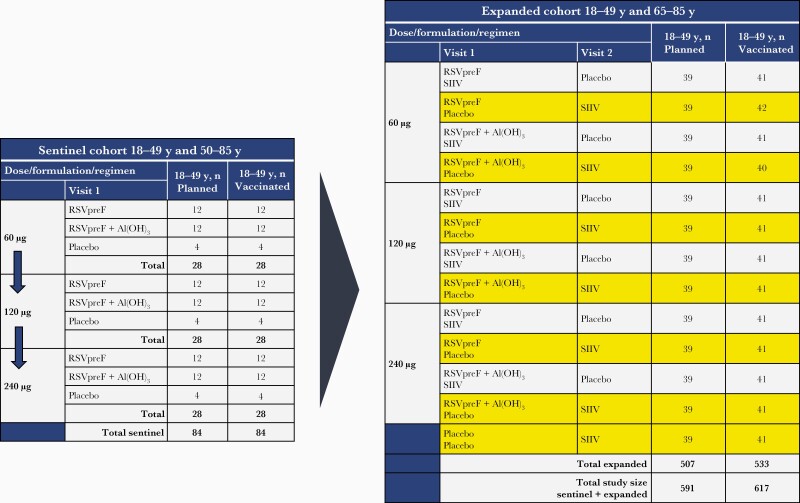
This phase 1/2 randomized, placebo-controlled, observer-blind, dose-finding study (NCT03529773) was conducted at 40 sites in the United States from April 2018 through November 2019. Three RSVpreF dose levels formulated with and without aluminum hydroxide (Al[OH]3) were evaluated in 2 phases (Figure 1). In phase 1, a sentinel cohort of up to approximately 168 participants 18–49 or 50–85 years old was randomized in a 1:3:3 ratio for each dose level and age stratum to receive placebo or RSVpreF with or without Al(OH)3. Randomization for the sentinel cohort began at the 60-µg dose level and proceeded stepwise to the 120- and 240-µg dose levels only after 14-day safety and tolerability data from the lower doses were deemed acceptable. In phase 2, an expanded cohort of up to approximately 1014 participants 18–49 or 65–85 years old were randomized (n = 507 per age group) to receive placebo or 1 of the dose levels and formulations, with or without seasonal inactivated influenza vaccine (SIIV). Expanded-cohort participants received 2 vaccinations approximately 1 month apart. Participants who received concomitant RSVpreF and SIIV at vaccination 1 received placebo at vaccination 2; those who received only RSVpreF or placebo at vaccination 1 received SIIV at vaccination 2. Safety of RSVpreF administered with SIIV for participants 18–49 years old, safety and immunogenicity results for participants 50–85 years old, and SIIV immune responses are presented in the separate report. Immunogenicity data from expanded cohort participants 18–49 years of age who received placebo are reported here and in the companion report. Additional details regarding study design and methods are in the Supplementary Material and study protocol, available at clinicaltrials.gov. Pfizer Inc was involved in study design and data collection, analysis, and interpretation. All authors had access to the data and attest to data accuracy and completeness.
Figure 1.
Study design. Expanded cohort groups included in this report are highlighted in yellow. Abbreviations: RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine; SIIV, seasonal inactivated influenza vaccine.
Participants
Participants were healthy adult men and nonpregnant women 18–49 years old who were required to use effective contraceptive methods or be otherwise unable to father or bear children, able to comply with study procedures, and provided written informed consent. The Supplementary Material lists exclusion criteria.
Randomization and Masking
In both phases, participants were randomized by an interactive response technology system. Participants, study and sponsor team members, and laboratory personnel performing immunologic assays were blinded as appropriate. Site dispensers and administrators were unblinded because the administered vaccines’ physical appearance differed.
Procedures
Each RSVpreF formulation contained equal amounts of recombinant RSV prefusion F trimer from subgroups A (Ontario) and B (Buenos Aires), totaling 60, 120, or 240 µg, formulated with or without 0.2 mg Al(OH)3. Lyophilized RSVpreF was reconstituted with either sterile water or Al(OH)3 in water as diluent and administered in a 0.5-mL injection volume; sentinel and expanded cohort participants receiving the vaccine were injected into the left deltoid. In the sentinel cohort, placebo (0.9% sodium chloride) was injected into the left deltoid. In the expanded cohort, placebo or SIIV (Fluzone Quadrivalent; Sanofi Pasteur) was injected into the right deltoid at vaccination 1 and into the deltoid of the nondominant arm at vaccination 2.
Outcomes
The primary study objective was to describe safety and tolerability of RSVpreF. Safety end points included local reactions and systemic events as reported by electronic diary within 14 days postvaccination 1 (see Supplementary Material for details; Supplementary Table 1), adverse events (AEs) within 1 month postvaccination 1 (sentinel and expanded cohort) and postvaccination 2 (expanded cohort), and medically attended AEs (MAEs) and serious AEs (SAEs) through 12 months postvaccination 1. Safety data were analyzed according to investigational product received. The safety population included all participants who received a dose of investigational product. The current report includes safety data from the sentinel and expanded cohorts combined.
Secondary study objectives included describing immune responses elicited by RSVpreF. Corresponding secondary end points included RSV A and RSV B neutralizing titers measured at prespecified time points through 6 months postvaccination 1. RSV neutralizing titers corresponding to a 100-µg/mL palivizumab serum concentration, which was associated with protection of high-risk infants from intensive care admissions for RSV disease in the pivotal IMPACT study (leading to licensure of palivizumab [38]), were also determined. Pfizer participated in the interlaboratory studies that assessed the first World Health Organization (WHO) International Standard for Antiserum to RSV (16/284; National Institute for Biological Standards and Control, Hertfordshire, United Kingdom) in our neutralization assays for RSV A [39] and RSV B [40]. We report a neutralization geometric mean titer (GMT) for the first WHO International Standard of 5300 for RSV A and 5632 for RSV B. To approximate WHO International Units/mL, Pfizer neutralization titers may be multiplied by the conversion factor of 0.377 for RSV A and 0.355 for RSV B. The Supplementary Material provides additional details regarding immunogenicity assays, exploratory objectives, and evaluable population.
This report includes safety and immunogenicity data from the sentinel and expanded cohorts combined according to investigational product received, but not for groups coadministered SIIV, which are reported separately.
Statistical Analysis
The sample size was not based on formal statistical hypothesis testing; safety and immunogenicity analyses were descriptive. Safety end points were evaluated descriptively using counts and percentages with 2-sided 95% confidence intervals (CIs). For immunogenicity analyses, GMTs at each time point and geometric mean fold rises (GMFRs) from prevaccination to each available time point postvaccination 1 were calculated for each group or subgroup. For GMTs and GMFRs, 95% CIs were calculated using a Student t distribution of the log-transformed data followed by back transformation to the original scale. Missing safety and immunogenicity data were not imputed.
RESULTS
Study Participants
A total of 618 participants 18–49 years old were recruited in the sentinel (n = 84) and expanded (n = 534) cohorts. Demographic characteristics were similar across cohorts and dose levels (Supplementary Tables 2 and 3).
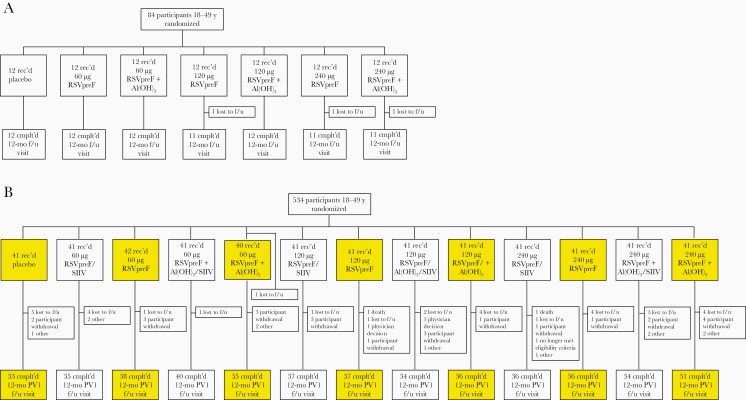
In the sentinel cohort, all but 2 participants completed the 1-month follow-up visit (Figure 2A). In the expanded cohort, 533 of 534 randomized participants were vaccinated, 523 completed the 1-month postvaccination 1 visit, and 462 completed the 12-month visit (Figure 2B). Withdrawals primarily resulted from loss to follow-up and participant withdrawal; no withdrawals were due to AEs.
Figure 2.
Disposition of participants 18–49 years old in the (A) sentinel and (B) expanded cohorts. Expanded cohort groups included in this report are highlighted in yellow. Participants who did not receive SIIV concomitantly with the RSV vaccine received it 1 month later. Abbreviations: Cmplt’d, completed; f/u, follow-up; PV1, postvaccination 1; rec’d, received; RSV, respiratory syncytial virus; RSVpreF, bivalent RSV prefusion F vaccine; SIIV, seasonal inactivated influenza vaccine.
Safety
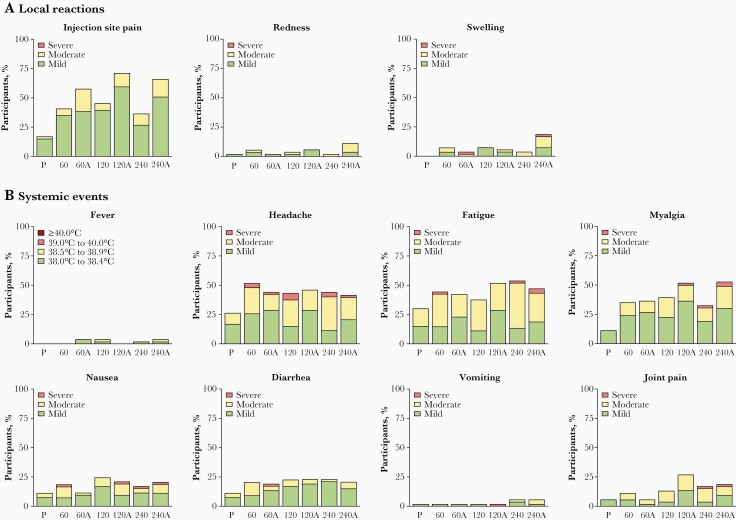
Safety data from the sentinel cohort supported decisions to move to higher dose levels and to the expanded cohort and are combined with expanded cohort data for ease of presentation except where noted. Local reactions were reported by 38.5%–71.2% of RSVpreF recipients per group and 18.9% of placebo recipients (Figure 3A). Local reactions were more frequent in RSVpreF + Al(OH)3 groups but did not differ by dose level. Most reported local reactions were mild or moderate; 1 participant in each of the 60-µg RSVpreF + Al(OH)3 and 240-µg RSVpreF + Al(OH)3 groups reported severe swelling. Median durations of individual local reactions were 1.0–6.0 days per group.
Figure 3.
Percentages of participants 18–49 years old in the sentinel and expanded cohorts combined reporting individual (A) local reactions or (B) systemic events by severity within 14 days postvaccination 1. Total number of participants (n) = 52–54 per group. Abbreviations: 60, 60 µg RSVpreF; 60A, 60 µg RSVpreF + AI(OH)3; 120, 120 µg RSVpreF; 120A, 120 µg RSVpreF + AI(OH)3; 240, 240 µg RSVpreF; 240A, 240 µg RSVpreF + AI(OH)3; P, placebo; RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine.
Systemic events were reported by 62.3%–77.4% of RSVpreF recipients per group and 47.2% of placebo recipients. The most commonly reported systemic events were headache (41.5%–51.9% of RSVpreF recipients per group vs 26.4% of placebo recipients), fatigue (37.7%–53.8% vs 30.2%), and myalgia (32.7%–52.8% vs 11.3%; Figure 3B). Most systemic events were mild or moderate with median durations of 1.0–10.0 days; the smaller number of severe systemic events were not dominated by any reaction type or associated with any specific treatment group. Seven RSVpreF recipients reported fevers, all of which were < 39.0°C and lasted a median of 1.0–5.0 days across groups.
Adverse events through 1 month postvaccination 1 were reported by 11.3%–21.2% of RSVpreF recipients per group and 9.4% of placebo recipients (Supplementary Table 4). Infections and infestations were generally the most common system organ class of reported AEs. Related AEs were reported by 0%–5.6% of RSVpreF recipients per group. Through 12 months postvaccination 1, SAEs were reported by 0%–5.7% of RSVpreF recipients per group and 1.9% of placebo recipients; none were considered related to investigational product. MAEs were reported by 13.5%–22.6% of RSVpreF recipients across groups; 1 was related (exanthem on the abdomen and arm). Eleven placebo recipients (20.8%) reported MAEs. Frequencies of reported AEs, SAEs, related AEs, and MAEs in the expanded cohort did not appreciably vary between groups (Supplementary Table 5). One participant in the 120-µg RSVpreF group died within 12 months postvaccination 1 due to toxicity to various agents (quetiapine and amlodipine) that was considered not vaccine-related.
Immunogenicity
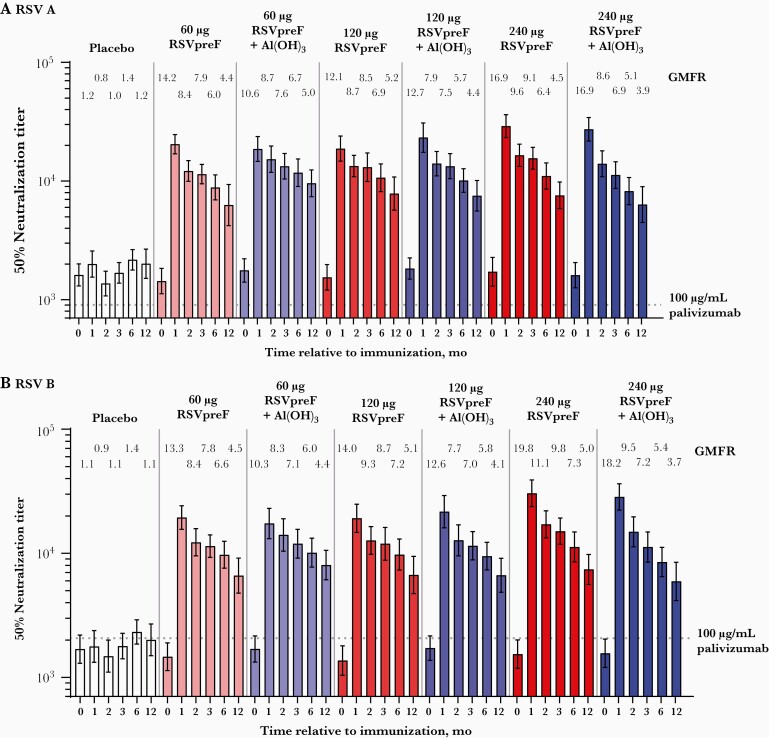
RSV A 50% neutralizing GMTs in RSVpreF formulation recipients in the combined sentinel and expanded cohorts rose from 1434–1828 prevaccination to 18 623–29 009 at 1 month postvaccination 1 (Figure 4A); comparatively, the RSV A neutralizing titer corresponding to a 100-μg/mL serum level of palivizumab is 903. For RSV B, GMTs rose from 1366–1718 to 17 374–30 443 (Figure 4B); the RSV B neutralizing titer corresponding to a 100-μg/mL palivizumab serum level is 2069. Corresponding neutralizing titer GMFRs were 10.6–16.9 for RSV A and 10.3–19.8 for RSV B. GMFRs trended higher in the 240-µg RSVpreF groups, and inclusion of Al(OH)3 did not appear to impact the immune response at any dose level. RSV A and RSV B GMFRs among placebo recipients were 1.2 and 1.1, respectively. The geometric mean ratio of combined RSV A/B neutralizing titer fold rise to anti-prefusion F immunoglobulin G (IgG) fold rise among all RSVpreF recipients was approximately 0.68 (Supplementary Figure 1).
Figure 4.
RSV neutralizing GMTs through 12 months postvaccination 1 and corresponding GMFRs compared with baseline for (A) RSV subgroup A and (B) RSV subgroup B in participants 18–49 years old in the sentinel and expanded cohorts combined. Data are shown separately by dose level for ease of viewing. Abbreviations: GMFR, geometric mean fold rise; GMT, geometric mean titer; RSV, respiratory syncytial virus; RSVpreF, bivalent RSV prefusion F vaccine.
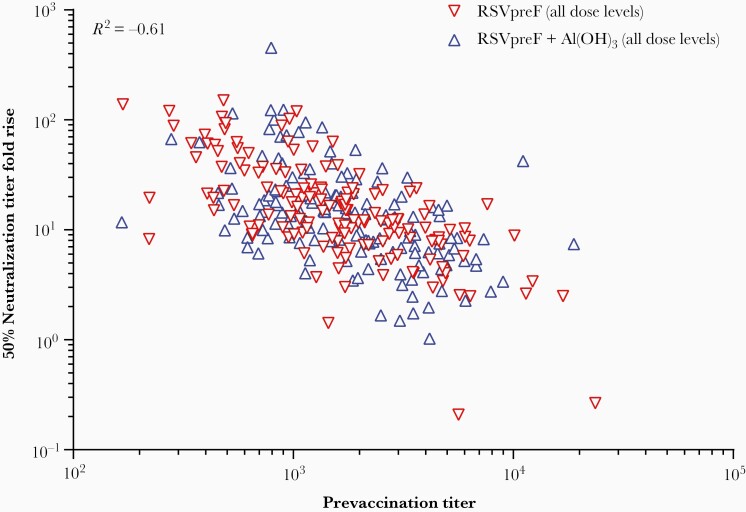
Neutralizing GMTs tended to be slightly higher among women than men, with corresponding GMFRs of 10.8–19.6 versus 8.9–14.2 for RSV A and 10.3–20.6 versus 8.5–18.0 for RSV B (Supplementary Figure 2). At 1 month postvaccination, GMFRs showed a steep inverse dependence on baseline titers (correlation, −0.61); individuals with the lowest baseline titers had the greatest vaccine GMFRs (Figure 5). Prefusion RSV F IgG subclass 1 (IgG1) GMFRs among women were 18.0–30.0 for RSV A in RSVpreF groups versus 1.1 in the placebo group at 1 month postvaccination 1; for RSV B, GMFRs were 16.4–30.6 versus 1.1 (Supplementary Figure 3).
Figure 5.
Vaccine-elicited RSV A/B neutralizing titer fold rise as a function of prevaccination RSV A/B neutralizing titers. For each individual, the prevaccination titer is the combined A/B 50% neutralizing titer, calculated as the geometric mean of the RSV A and B neutralizing titers. The combined A/B 50% neutralizing titer fold rise is the geometric mean of the fold rises of 50% neutralizing titers against RSV A and RSV B from prevaccination to 1 month postvaccination. The correlation across all data was calculated at −0.61. Data are shown for participants 18–49 years old in the sentinel and expanded cohorts combined. Abbreviations: GMFR, geometric mean fold rise; RSV, respiratory syncytial virus; RSVpreF, bivalent RSV prefusion F vaccine.
Serum neutralizing titers remained substantially above baseline through 12 months postvaccination 1 (Figure 4). Neutralizing titer GMFRs at 12 months for RSV A were 3.9–5.2 across RSVpreF groups versus 1.2 for placebo and 3.7–5.1 versus 1.1, respectively, for RSV B.
DISCUSSION
Despite decades of vaccine-development efforts, protection against RSV disease remains an unmet global need [5]. Intranasally administered live attenuated vaccines have not been associated with RSV disease enhancement; however, achieving the proper balance of immunogenicity and reactogenicity in infants has been challenging [21]. An extended half-life prophylactic monoclonal antibody, nirsevimab, is in development for direct protection of infants [41]. Maternal immunization aims to elicit a polyclonal response to passively protect infants against RSV at the age of greatest risk [3, 21].
In this study, RSVpreF, a bivalent vaccine candidate including F antigens from subgroups A and B, showed a benign safety and tolerability profile and elicited robust neutralizing responses against both subgroups in participants 18–49 years old. Local reactions and systemic events were generally mild or moderate across vaccine groups, although local reaction rates were higher with Al(OH)3-containing formulations. There were no vaccine-related SAEs.
Serum neutralizing titer GMFRs from prevaccination to 1 month postvaccination elicited by an intramuscular RSVpreF dose were 10–20, far greater than the GMFRs of approximately 2–4-fold elicited by F not stabilized in the prefusion conformation in previous trials [19, 37]. Additionally, RSVpreF elicited robust neutralizing responses against both RSV A and B subgroups in contrast with another investigational RSV prefusion F-based vaccine [35], which is important because RSV subgroup dominance varies over time [42]. These data confirm that prefusion F elicits far greater neutralizing responses in humans than postfusion or structurally undefined F [35] and that a bivalent vaccine candidate elicits a more balanced neutralizing response across subgroups. The postvaccination serum neutralizing GMTs were approximately 10-fold greater than those corresponding to the highly protective 100-μg/mL serum level of palivizumab [38]. The similarity of RSV neutralization titers of placebo recipients’ sera to those of a protective palivizumab concentration is consistent with the low incidence of severe RSV disease in healthy young adults and the increase in RSV disease in infants after 1 or 2 maternal antibody half-lives [43–45]. Immune responses trended toward a shallow dose-level response, with highest neutralizing GMTs and GMFRs elicited by the 240-µg dose level. Adsorption to Al(OH)3 did not generally appear to enhance neutralizing responses to RSVpreF, consistent with other RSV investigational vaccines [35, 46].
Several additional findings are particularly promising for using RSVpreF for maternal immunization. Slightly higher immune responses were elicited in women than men, similar to other vaccines [47]. Because neutralizing GMFRs were much higher for women with low versus high baseline titers, infants most in need of transplacentally transferred neutralizing antibody will likely receive the most benefit from maternal immunization. While neutralizing GMFRs declined through 12 months postvaccination, an important finding was that GMFRs were still 4–5 fold higher than prevaccination at 12 months. These data are encouraging as immunization in the late second or third trimester may result in substantially elevated titers through delivery. High IgG1 antigen-binding GMFRs indicate that RSVpreF elicits the antibody subclass most efficiently transferred across the placenta to the fetus [48]. Additionally, the approximately 0.68 ratio of RSV A/B neutralizing titer fold rise to vaccine antigen-binding IgG fold rise for all formulations and dose levels combined suggests that most of the vaccine-elicited antibody can neutralize RSV. As described previously, greater elicitation of RSV neutralizing relative to F binding activity may increase the likelihood of vaccine efficacy and decrease the likelihood of vaccine-mediated disease enhancement [35]. The high ratio observed in this study contrasts with the < 0.25 ratio for a monovalent nonprefusion F vaccine [49], which did not meet prespecified primary efficacy end points in a pivotal phase 3 study [19].
Study strengths include its large size and evaluation of multiple vaccine formulations. By relating vaccine-elicited serum neutralizing titers to those associated with protective palivizumab levels in high-risk infants, the serological findings presented here are linked to a potential biomarker for efficacy. A study limitation is the lack of power for statistical comparisons across groups. Ongoing studies will further inform use of RSVpreF for maternal immunization. A subset of participants from this study will be revaccinated 1 year after initial vaccination; resulting safety and immunogenicity data will inform repeated immunizations potentially required for women with multiple pregnancies.
Additional adjuvanted formulations of RSVpreF are being evaluated in a phase 1/2 study in older adults (NCT03572062). The RSVpreF maternal immunization program also includes a Tdap concomitant use phase 2b study (NCT04071158) in nonpregnant women 18–49 years old and a phase 2b safety and immunogenicity study (NCT04032093) in pregnant women and their infants. A pivotal phase 3 efficacy study in pregnant women and their infants (NCT04424316) is currently underway using the 120-µg dose without Al(OH)3, as informed by this and the phase 2b study in nonpregnant women [50].
Overall, this phase 1/2 study showed different formulations and dose levels of the RSVpreF investigational vaccine to be safe, well tolerated, and highly immunogenic in adults 18–49 years of age. The findings support continued RSVpreF development for maternal vaccination to protect young infants from RSV disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank those who participated in the study and those who conducted the study, including nurses, physicians, coordinators, clinical scientists, laboratory personnel, and other critical colleagues. We also thank the following individuals at Pfizer Inc: Katie McDonald, Christine Juergens, Qin Jiang, Mary Beth Turner, Han-Qing Jiang, Luke Cunliffe, Naren Surampalli, Michelle McLean, and Keri Clarke at SRG; and the following individuals at the University of Rochester: Angela R. Branche, Emily Pierce, Mary Criddle, Sally Thomas, and Doreen Francis. Editorial/medical writing support was provided by Judith Kandel, PhD, Emily Stackpole, PhD, and Allison Gillies, PhD, of ICON (North Wales, PA) and was funded by Pfizer Inc.
Disclaimer . Pfizer Inc was involved with study concept and design; collection, analysis, and interpretation of the data; drafting of the manuscript; and the decision to submit the manuscript for publication. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Financial support. This work was supported by Pfizer Inc.
Potential conflicts of interest. E. E. W. reports grants from Merck, Pfizer Inc, and Janssen; and has served as an unpaid consultant to Novavax, Merck, GlaxoSmithKline, and Janssen. A. R. F. reports grants from Merck, Pfizer Inc, BioFire Diagnostics, and Janssen; and serves on a Data and Safety Monitoring Board for Novavax. All other authors are employees of Pfizer Inc and may hold stock and/or stock options.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek 2019 conference, Washington, DC, 2–6 October 2019; and RSVVW 2019 conference in Accra, Ghana, 12–14 November 2019.
Data sharing statement. Upon request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union, or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1. Hall CB, Simoes EAF, Anderson LJ.. Clinical and epidemiologic features of respiratory syncytial virus. In: Anderson LJ, Graham BS, eds. Challenges and opportunities for respiratory syncytial virus vaccines. Berlin, Heidelberg: Springer, 2013:39–57. [Google Scholar]
- 2. Glezen WP, Taber LH, Frank AL, Kasel JA.. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
- 3. Murray J, Bottle A, Sharland M, et al. . Risk factors for hospital admission with RSV bronchiolitis in England: a population-based birth cohort study. PLoS One 2014; 9:e89186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rha B, Curns AT, Lively JY, et al. . Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics 2020; 146:e20193611. [DOI] [PubMed] [Google Scholar]
- 5. Boyoglu-Barnum S, Chirkova T, Anderson LJ.. Biology of infection and disease pathogenesis to guide RSV vaccine development. Front Immunol 2019; 10:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson S, Oliver C, Prince GA, et al. . Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis 1997; 176:1215–24. [DOI] [PubMed] [Google Scholar]
- 7. The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 8. Meissner HC, Kimberlin DW.. RSV immunoprophylaxis: does the benefit justify the cost? Pediatrics 2013; 132:915–8. [DOI] [PubMed] [Google Scholar]
- 9. AAP Committee on Infectious Diseases. Red Book® 2018-2021. 31st ed. Itasca, IL: American Academy of Pediatrics, 2018. [Google Scholar]
- 10. Kim HW, Canchola JG, Brandt CD, et al. . Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–34. [DOI] [PubMed] [Google Scholar]
- 11. Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE.. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89:405–21. [DOI] [PubMed] [Google Scholar]
- 12. Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G.. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 1969; 89:435–48. [DOI] [PubMed] [Google Scholar]
- 13. Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH.. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 1969; 89:449–63. [DOI] [PubMed] [Google Scholar]
- 14. Domachowske JB, Rosenberg HF.. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev 1999; 12:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waris ME, Tsou C, Erdman DD, Day DB, Anderson LJ.. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. J Virol 1997; 71:6935–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL.. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98:708–15. [DOI] [PubMed] [Google Scholar]
- 17. Ochola R, Sande C, Fegan G, et al. . The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One 2009; 4:e8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu HY, Steinhoff MC, Magaret A, et al. . Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J Infect Dis 2014; 210:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madhi SA, Polack FP, Piedra PA, et al. . Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 2020; 383:426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munoz FM, Swamy GK, Hickman SP, et al. . Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J Infect Dis 2019; 220:1802–15. [DOI] [PubMed] [Google Scholar]
- 21. Aranda SS, Polack FP.. Prevention of pediatric respiratory syncytial virus lower respiratory tract illness: perspectives for the next decade. Front Immunol 2019; 10:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women--Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–5. [PMC free article] [PubMed] [Google Scholar]
- 23. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB.. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018-19 influenza season. MMWR Recomm Rep 2018; 67:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baxter R, Bartlett J, Fireman B, Lewis E, Klein NP.. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics 2017; 139:e20164091. [DOI] [PubMed] [Google Scholar]
- 25. Singh A, Pallikadavath S, Ogollah R, Stones W.. Maternal tetanus toxoid vaccination and neonatal mortality in rural north India. PLoS One 2012; 7:e48891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine S, Klaiber-Franco R, Paradiso PR.. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol 1987; 68:2521–4. [DOI] [PubMed] [Google Scholar]
- 27. Wertz GW, Collins PL, Huang Y, Gruber C, Levine S, Ball LA.. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci USA 1985; 82:4075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLellan JS, Chen M, Leung S, et al. . Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013; 340:1113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson PR, Spriggs MK, Olmsted RA, Collins PL.. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci USA 1987; 84:5625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson PR, Collins PL.. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol 1988; 69:2623–8. [DOI] [PubMed] [Google Scholar]
- 31. Hause AM, Henke DM, Avadhanula V, Shaw CA, Tapia LI, Piedra PA.. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS One 2017; 12:e0175792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calder LJ, Gonzalez-Reyes L, Garcia-Barreno B, et al. . Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology 2000; 271:122–31. [DOI] [PubMed] [Google Scholar]
- 33. Smith BJ, Lawrence MC, Colman PM.. Modelling the structure of the fusion protein from human respiratory syncytial virus. Protein Eng 2002; 15:365–71. [DOI] [PubMed] [Google Scholar]
- 34. McLellan JS, Chen M, Joyce MG, et al. . Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013; 342:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crank MC, Ruckwardt TJ, Chen M, et al. . A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019; 365:505–9. [DOI] [PubMed] [Google Scholar]
- 36. McLellan JS. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr Opin Virol 2015; 11:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Falloon J, Yu J, Esser MT, et al. . An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017; 216:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forbes ML, Kumar VR, Yogev R, Wu X, Robbie GJ, Ambrose CS.. Serum palivizumab level is associated with decreased severity of respiratory syncytial virus disease in high-risk infants. Hum Vaccin Immunother 2014; 10:2789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McDonald JU, Rigsby P, Dougall T, Engelhardt OG, Study Participants. Establishment of the first WHO international standard for antiserum to respiratory syncytial virus: report of an international collaborative study. Vaccine 2018; 36:7641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDonald JU, Rigsby P, Atkinson E, Engelhardt OG, Study Participants. Expansion of the 1st WHO international standard for antiserum to respiratory syncytial virus to include neutralisation titres against RSV subtype B: an international collaborative study. Vaccine 2020; 38:800–7. [DOI] [PubMed] [Google Scholar]
- 41. Griffin MP, Yuan Y, Takas T, et al. . Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020; 383:415–25. [DOI] [PubMed] [Google Scholar]
- 42. Hall CB, Walsh EE, Schnabel KC, et al. . Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis 1990; 162:1283–90. [DOI] [PubMed] [Google Scholar]
- 43. Parikh RC, McLaurin KK, Margulis AV, et al. . Chronologic age at hospitalization for respiratory syncytial virus among preterm and term infants in the United States. Infect Dis Ther 2017; 6:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hall CB, Long CE, Schnabel KC.. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis 2001; 33:792–6. [DOI] [PubMed] [Google Scholar]
- 45. Sarvas H, Seppala I, Kurikka S, Siegberg R, Makela O.. Half-life of the maternal IgG1 allotype in infants. J Clin Immunol 1993; 13:145–51. [DOI] [PubMed] [Google Scholar]
- 46. Langley JM, Sales V, McGeer A, et al. . A dose-ranging study of a subunit respiratory syncytial virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults ≥65 years of age. Vaccine 2009; 27:5913–9. [DOI] [PubMed] [Google Scholar]
- 47. Flanagan KL, Fink AL, Plebanski M, Klein SL.. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol 2017; 33:577–99. [DOI] [PubMed] [Google Scholar]
- 48. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M.. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012:985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Glenn GM, Fries LF, Thomas DN, et al. . A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis 2016; 213:411–22. [DOI] [PubMed] [Google Scholar]
- 50. Peterson JT, Zareba AM, Fitz-Patrick D, et al. . Safety and immunogenicity of a respiratory syncytial virus prefusion F vaccine when co-administered with a tetanus, diphtheria, and acellular pertussis vaccine [published online ahead of print 12 October 2021]. J Infect Dis doi: 10.1093/infdis/jiab505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.