Abstract
Ascorbic acid (AsA) also known as vitamin C is considered as an essential micronutrient in the diet of humans. The human body is unable to synthesize AsA, thus solely dependent on exogenous sources to accomplish the nutritional requirement. AsA plays a crucial role in different physiological aspects of human health like bone formation, iron absorption, maintenance and development of connective tissues, conversion of cholesterol to bile acid and production of serotonin. It carries antioxidant properties and is involved in curing various clinical disorders such as scurvy, viral infection, neurodegenerative diseases, cardiovascular diseases, anemia, and diabetes. It also plays a significant role in COVID-19 prevention and recovery by improving the oxygen index and enhancing the production of natural killer cells and T-lymphocytes. In plants, AsA plays important role in floral induction, seed germination, senescence, ROS regulation and photosynthesis. AsA is an essential counterpart of the antioxidant system and helps to defend the plants against abiotic and biotic stresses. Surprisingly, the deficiencies of AsA are spreading in both developed and developing countries. The amount of AsA in the major food crops such as wheat, rice, maize, and other raw natural plant foods is inadequate to fulfill its dietary requirements. Hence, the biofortification of AsA in staple crops would be feasible and cost-effective means of delivering AsA to populations that may have limited access to diverse diets and other interventions. In this review, we endeavor to provide information on the role of AsA in plants and human health, and also perused various biotechnological and agronomical approaches for elevating AsA content in food crops.
Keywords: Ascorbic acid, Vitamin C, Antioxidant, COVID-19, GDP-L-galactose phosphorylase Smirnoff-Wheeler pathway, Stress
Introduction
L- Ascorbic acid (AsA) is the water-soluble active form of vitamin C found in plants (Mitmesser et al. 2016; Akram et al. 2017). It is present ubiquitously in both plants and animals but absent in prokaryotes. Humans, unlike other animals, are unable to synthesize AsA due to the non-functional L-gulono 1–4 lactone oxidase of the AsA biosynthesis pathway. Therefore, human’s nutritional source of AsA is mainly dependent on plant foods. The primary dietary sources of AsA are tubers, citrus fruits, kiwi, nuts, vegetables, sprouts, capsicum, guava and strawberries (Maruta et al. 2010; Visser et al. 2011). Recommended Dietary Allowance (RDA) of AsA in males and females is 75 and 90 mg/day, respectively (Paciolla et al. 2019). Smokers need an extra 35 mg/day due to oxidative stress (Ueta et al. 2003). Around 23% of the total world population is not consuming the RDA intake of AsA. Nearly 7.9% of the USA population were found to be clinically AsA deficient (Rowe and Carr 2020). A study conducted on a group of Indian populations had shown variable AsA deficiency from 45.7–73.9% (Ravindran et al. 2011).
In humans, AsA is acclaimed for preventing scurvy, a fatal disease of erstwhile (Chambial et al. 2013), though it is seldom nowadays and about 7% of people show latent scurvy with symptoms like weakness and low energy (Macknight et al. 2017). AsA plays two noteworthy roles in cells. Firstly, it acts as an antioxidant and cofactor for dioxygenase and monooxygenase enzymes (Padayatty et al. 2017; Smirnoff 2018a). These enzymes have a major role in epigenetic DNA demethylation (Smirnoff 2018a). Embryonic development, cancer progression and ageing-related factors are linked with epigenetic programming and ascorbate influences all these processes. Secondly, at low concentration, AsA acts as an electron donor and produce free radicals (Smirnoff 2018a). A well-characterized pro-oxidant effect of AsA is the reduction of Fe3+ and Cu2+ and the production of free hydroxyl radicals of Fe2+/Cu+ and H2O2 by Fenton reaction (Padayatty et al. 2017). Fe2+ further reduce the oxygen into superoxides and H2O2. The report showed that the toxicity of AsA in mammalian cell culture or culture media is high when AsA is present in a low amount (Przyby 2020). Nearly two billion people worldwide are suffering from iron deficiency anemia (IDA) (Miller 2013). AsA promotes the uptake of non-heme iron and enhances iron absorption in the body. AsA stabilizes the folate in food and plasma which leads to increased excretion of oxidized folate derivatives, and absorption of non-heme iron (Golding 2018). For plants, iron is an essential micronutrient as it acts as a redox cofactor in photosynthesis and respiration (Grillet et al. 2014). This property of iron makes it toxic when present in excess as it catalyzes the formation of free radicals. Although present in abundance in the Earth's crust it is not readily available to plants due to its poorly soluble form i.e. Fe(III). Plants have evolved two strategies to reduce Fe(III) form into Fe(II) for its uptake (Grillet et al. 2014; Smirnoff 2018b). However, iron deficiency anaemia (IDA) is a major nutrient deficiency in developing countries. It is necessary to increase iron content to combat IDA through agricultural improvement. It has been reported that AsA increases the iron accumulation in Arabidopsis seeds (Grillet et al. 2014). Iron accumulation in sink tissue is mediated by the ascorbate efflux. Hence, up-regulation of AsA can not only enhance ascorbic acid accumulation but also elevate iron content in plants (Grillet et al. 2014). AsA, an antioxidant varies in its content among plants and different tissues of the same plant. Most of the staple crops carry a low amount of AsA thus, there is a need to have food crops having plentiful AsA to curb malnutrition (Fig. 1). Malnutrition or hidden hunger is a major problem in developing and underdeveloped countries due to the limited access of diverse diets and other nutrient interventions. In these countries, the population is predominantly dependent on staple food crops which are rich in carbohydrates but low in micronutrients (Chaturvedi et al. 2021). Thus, biofortification of staple crops through agronomic approach, genetic engineering and conventional breeding could be the possible way to eradicate malnutrition from the world (Chaturvedi et al. 2021). Biofortification of crops also aids in plant health and production, reducing the abiotic and biotic stress conditions. AsA biofortified crops had shown improved tolerance against different oxidative stresses and this also gives a possible way to maintain productivity during global climate change (Akram et al. 2017; Boubakri 2017).
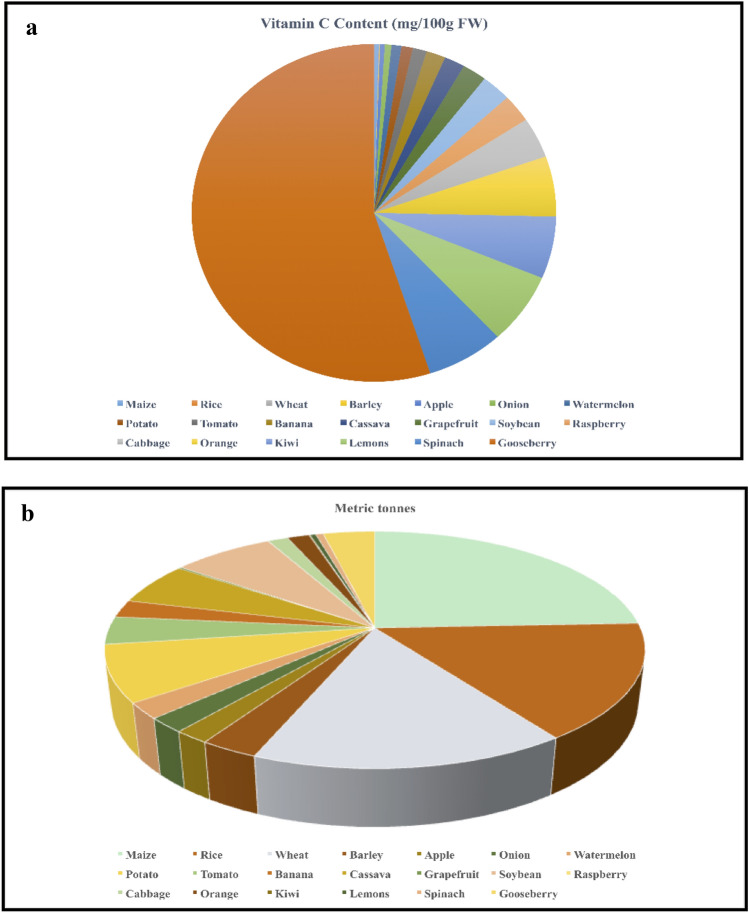
Fig. 1.
Representation of major crop production in the year 2020 in the world followed by their AsA content (mg/100 g fresh weight (F.W.). 1a) Production (metric tons) status of major staple food crops viz., maize, wheat, and rice along with other crops. 1b) Food crops showing the AsA content. Staple crops (maize, wheat rice) showed the lesser amount of AsA, while the highest amount of AsA was shown in spinach followed by lemon and kiwi. The data of major crop production
adapted from the food and agriculture organization (FAO 2020, http://www.fao.org/faostat/en/#data/QC/visualize) and AsA content taken from the USDA (https://www.nal.usda.gov/) composition database
Role of AsA in plants
About 1% of available glucose is utilized for AsA production in plants (Paciolla et al. 2019). AsA is ubiquitously present in all the cells of plants, including cell wall, extracellular matrix, and apoplast (Macknight et al. 2017). AsA content differs significantly between plant species and plant organs. A higher amount of AsA is found in developing tissues, and the lowest amount is present in the roots (Smirnoff 2018b; Paciolla et al. 2019). Plant developmental and environmental changes influence AsA content in organs and tissues. Light is an essential environmental signal in regulating AsA levels in plants (Yabuta et al. 2007; Heyneke et al. 2013). It has been observed that in the sprouts of Vigna radiata, the AsA level has increased to 21.4-fold in semi-light and 29.8-fold after three days of full light exposure (Lu and Guo 2020). Different wavelengths of lights showed a different pattern in AsA synthesis. Variation in the AsA content significantly altered the expression of genes linked with photosynthesis. AsA is a cofactor of enzyme violaxanthin de-epoxidase, thus plays a role in the synthesis of xanthophylls. Hence, it is vital for the protection of photosystem II (PSII) by dissipating excess excitation energy in the light-harvesting complexes (Saga et al. 2010; Awad et al. 2015; Paciolla et al. 2019). In tomatoes, exogenous application of AsA increased the fruit ripening (Steelheart et al. 2020). The AsA deficient mutant of Arabidopsis showed earlier senescence compared to wild-type plants (Kiddle et al. 2003). AsA is the cofactor of enzyme 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase and catalyzes the last rate-limiting step of the ethylene biosynthetic pathway (Smirnoff 2018b). It also acts as a cofactor for the biosynthesis pathways of gibberellins, abscisic acid, and in the catabolism of auxins (Akram et al. 2017). AsA plays role in mitigating plant pathogen infection and its defense mechanism is dependent on pathogen lifestyles. In plants, the biotrophic pathogens infection is usually controlled through salicylic acid (SA) signaling. However, for necrotrophic pathogens, it is arbitrated through jasmonic acid and ethylene signaling (Pastori et al. 2003; Glazebrook 2005; Akram et al. 2017). Various reports showed that Arabidopsis mutants with low AsA showed the high depositions of SA, pathogenesis-related proteins, and camalexin. These mutants were more resistant against biotrophic pathogen Pseudomonas syringae and Peronospora parasitica. While on the other hand, they were highly susceptible to necrotrophic ascomycetes Alternaria brassicicola (Egan et al. 2007; Botanga et al. 2012). Nonetheless, the exogenous addition of AsA induces resistance against different plant-pathogen interactions. AsA controls cell division, elongation, differentiation and programmed cell death (PCD) (Bellini and De Tullio 2019). In presence of AsA, meristematic cells of the root can reduce the length of the G1 phase and promote entry to the S phase of the cell cycle. Cell enriched with AsA has a higher cell division rate, on the contrary, a higher level of DHA causes the reduction of cell division which indicates that AsA fundamentally increased the cell cycle progression.
Smirnoff-wheeler (SW) pathway
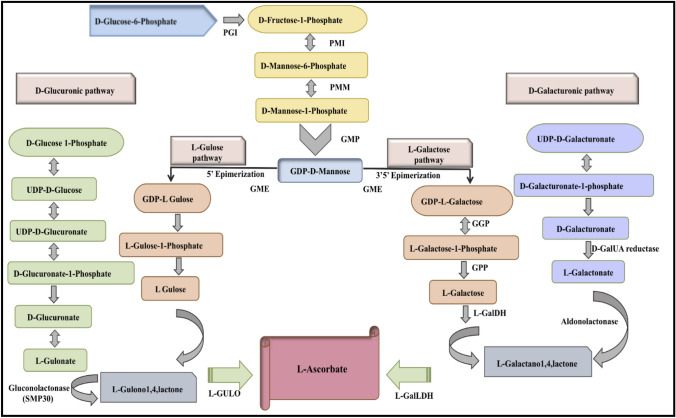
SW pathway (Fig. 2) is the predominant pathway for the synthesis of AsA in plants. It is compelled via the conversion of D-glucose-6-phosphate to D-fructose-6-phosphate by phosphoglucose isomerase (PGI) (Yabuta et al. 2007). D-fructose-6-phosphate is transformed to D-mannose-6-phosphate and D-mannose 1-phosphate catalyzed by phosphomannose isomerase (PMI) and phosphomannose mutase (PMM), respectively (Maruta et al. 2010). Further, GDP-D mannose pyrophosphorylase, a reversible enzyme, relocates guanosine monophosphate from GTP to D-mannose 1-phosphate and forms GDP-D-mannose. It was first identified by silencing map-based vitamin c deficient 1 (vtc1) mutant (At2g39770) (Conklin and Barth 2004). GDP-D-mannose is acted upon by GDP-D-mannose-3′, 5′-epimerase (GME) that catalyzes double epimerization. The first epimerization of 5'GDP-mannose by GME can produce GDP-L-gulose (Wolucka and Van Montagu 2003). GME shows feedback response because it is inhibited by the ascorbate, GDP, GDP-gulose and GDP-fucose. The first committed step of the SW pathway is the conversion of GDP-L-galactose to L-galactose-1-P and GDP by utilizing inorganic phosphate. This step is catalyzed by the GDP-l-galactose-phosphorylase (GGP) (Dowdle et al. 2007; Bulley and Laing 2016).
Fig. 2.
Pictorial representation of four different pathways for L-ascorbate biosynthesis in plants. These are the L-galactose or Smirnoff-wheeler (SW) pathway, L-gulose pathway, D-glucuronic pathway/ MIOX pathway and D-galacturonic pathway
Further, the galactose-1-phosphate is transformed into L-galactose and L-galatono-1,4-lactone via galactose-1-phosphate phosphatase (GPP) and L-galactose dehydrogenase (L-GalDH), respectively, where L-galatono-1,4-lactone is irreversibly oxidized by the L-galactono-1, 4-lactone dehydrogenase (L-GalLDH) to form ascorbate (Bulley and Laing 2016). All the enzymes of SW pathways are located in the cytosol, except L-GalLDH, which remains in the inner membrane of mitochondria.
Alternative AsA biosynthesis pathway in plants via L-gulose
In support of the D-mannose/L-galactose (D-Man/L-Gal) pathway, other alternative pathways are moving via D-glucuronic acid, L-gulose, and D-galacturonic acid. In the D-Man/L-Gal pathway, epimerization through GDP-D-mannose-3′,5′-epimerase (GME) leads to the formation of two discrete products i.e., GDP-L-galactose (GDP-L-Gal) and GDP-L-gulose (GDP-L-Gul). GDP-L-Gul is a rare sugar found in bacteria and algae but not a structural component of plants. This GDP-L-Gul is channelled directly in the ascorbate pathway through L-gulono-1,4-lactone.
D-glucuronic or MIOX pathway
In plants, AsA biosynthesis proceeds via GDP-Man, GDP-L-Gal, L-gal-1-phosphate, L-Gal, and L-GalL (Wheeler et al. 1998). Although the AsA biosynthetic pathway proposed by Smirnoff and Wheeler (2000) is most prominent, there are pieces of evidence that signify the existence of other pathways that drive plants to synthesize AsA. Additional proof of the complex network of AsA biosynthesis is to use myo-inositol (MI) as an ascorbate substrate. To investigate the role of MI, the fourth homologs of myo-inositol oxygenase (MIOX4) was constitutively overexpressed in Arabidopsis and showed a 2 to 3 fold increased ascorbate level as compared to control (Lorence et al. 2004). It was further supported by the overexpression of purple acid phosphatase (AtPAP) that increased the foliar AsA content in Arabidopsis (Zhang et al. 2008). The AtPAP role is predicted as a phytase that reacts on myo-inositol hexakisphosphate and produces MI and free phosphate. It is also assumed that MI is an AsA precursor (Zhang et al. 2008). Another study revealed that reduction in inositol- (1,4,5) trisphosphate led to the accumulation of AsA due to upregulation of MIOX pathway genes (Alimohammadi et al. 2012). The other study has shown the contradictory observation and revealed that MIOX controls the level of MI but did not influence the AsA concentration in Arabidopsis plants (Endres and Tenhaken 2009). This study is also supported by radiolabelling of 3H‐MI in Arabidopsis which revealed no role of the MI pathway in AsA synthesis (Ivanov Kavkova et al. 2019). A recent study on tomatoes showed that MIOX4 overexpression in tomatoes increases the AsA accumulation. It was further validated by the feeding method, which showed the involvement of the MIOX pathway in AsA biosynthesis (Munir et al. 2020). Hence, the rate-limiting genes of the MIOX pathway need to be explored further for their role in the AsA enhancement of crop plants.
Recycling pathway
In both plants and animals, the oxidized stable form of AsA is known as DHA. Oxidation of AsA converted to radicle monodehydroascorbate (MDHA) is spontaneously turned into DHA. MDHA and DHA can convert into AsA by the monodehydroascorbate reductase (MDHAR) and DHAR, respectively (Foyer and Halliwell 1976).
Importance of AsA in human health
AsA has a variety of functions in human health and development. It prevents protein, DNA, and lipids from oxidative damage by scavenging free radicals and acts as an antioxidant in humans (Smirnoff 2018a). For instance, 500 to 750 µM concentration of AsA helps to protect against oxidative damage to the eyes from solar radiation and also inhibits melanogenesis (Panich et al. 2011; Umapathy et al. 2013). AsA also plays role in the treatment of several skin-related diseases such as atopic dermatitis (AD), malignant melanoma, porphyria cutanea tarda (PCT), herpes zoster, and postherpetic neuralgia. AD is a chronic relapsing inflammation of the skin related to allergies. The lesions are characterized by erythematous papules with itching or scaling and are reported to affect 15–30% of the children population (Kim et al. 2013; Tollefson et al. 2014). It leads to structural and functional damage to the skin barrier. AsA helps in the differentiation and production of interstitial material and reduces chronic inflammation (Uchida et al. 2001). Another disease malignant melanoma derived from melanocytes is a kind of skin tumor that occurs in the skin and mucous membrane transfer (Yussif et al. 2017). AsA affects the function and quantity of melanocytes, thereby reducing the synthesis of melanocytes. The anti-melanogenesis effect of AsA is due to its role as a reducing agent in various oxidation stages of melanin formation (Yussif et al. 2017). Furthermore, AsA is also reported to reduce the melanogenesis of melanoma cells by stimulating the production of α-melanocyte-stimulating hormone (α-MSH) (Stojkovic‐Filipovic and Kittler 2014). AsA also helps in diminishing genetic diseases such as PCT, which is characterized by extreme photosensitivity that causes blistering on skin lesions. It is developed by the deficiency of enzyme uroporphyrinogen decarboxylase (UROD) which leads to excess production of uroporphyrin and heptacarboxylporphyrin by hepatic cells. Deficiency of AsA in plasma cells is one of the most prevalent reasons for the development of PCT. AsA inhibit catalytic oxidation of CYP1LA2, which is known to help in the production of uroporphyrin and heptacarboxylporphyrin. Besides maintaining skin health, AsA is also reported to involve in the treatment of cancer diseases. Further, the intravenous administration of ascorbate is reported to be a supportive intervention to increase the plasma concentration that also reduce the inflammation of chemotherapy during cancer treatment (Shenoy et al. 2018a). The role and mode of action of AsA in management of various diseases are mentioned in Table 1.
Table 1.
Role of AsA and their mode of actions in various diseases
| S. No | Concentration of AsA | AsA role in disease | Mode of actions of AsA on diseases | References |
|---|---|---|---|---|
| 1 | 41–66 µmol/L | Cardiac disease | Prevents the low-density lipoproteins (LDL-proteins) from oxidative changes, prevention of ischemia/reperfusion and myocardial injury ameliorated the toxicity of the heavy metals | He et al. (2007); Kukongviriyapan et al. (2014); Morelli et al. (2020) |
| 2 | 200 to 400 mg/kg | Alzheimer's disease | Reduced the depletion of dopamine and serotonin metabolites in cortex and striatum | Harrison (2012) |
| 3 | 150 mg/day | Prostate cancer | Inhibit the angiogenesis and increase the cytotoxicity in naïve human prostate cancer cell lines | Bai et al. (2015) |
| 4 | 75 g to 125 g | Pancreatic cancer | Increased the Bax/Bcl-2 expression, ROS production, DNA fragmentation and cytotoxicity of cells | Drisko et al. (2018) |
| 5 | 100 µg/ml | Cervical cancer | Increased the susceptibility of cisplatin and doxorubicin drug on HeLa cells and increase the level of apoptotic protein, catalase, superoxide dismutase and endoplasmic reticulum induced p-eIF2/eif2-a ratio | Shenoy et al. (2018b) |
| 6 | 50, 100 and 200 µM | Breast cancer | Induced the autophagosome by regulation of mammalian target of rapamycin (mTOR), Beclin1 and autophagy-related genes, accumulation of p62 protein, increased endoplasmic reticulum stress via IRE-JNK-CHOP signalling pathways | Bos (2019) |
| 7 | 0.5 mM, 2.5 mM, and 5 mM | Parkinson disease | Decreases the level of α-synuclein-Cu2 + and accumulation of Lewy bodies | da Costa Daniele et al. (2020) |
| 8 | 100 mg/kg to 4 g/kg | Colon cancer | Increases the cytotoxic effects of CD8 T cells, check the tumour growth by immune checkpoint therapy | Magrì et al. (2020) |
Coronavirus disease (COVID-19) is a pathogenic viral infection that first emerged in Wuhan city of China in December 2019 (Mittal et al. 2020). The most common symptoms of SARS-CoV2 are fever, dry cough, and tiredness, while the serious symptoms include shortness of breathing, chest pain and loss of speech or movement (Mittal et al. 2020). Most of the patients does not show severe symptoms however, nearly 5% of the patient showed serious lung injury, multiple organ failure and sepsis in the patient due to the infection (Marik et al. 2017). Severe infection of SARS-CoV2 leads to sepsis which is caused by cytokine storms in the body after the severe immune reaction. Various studies suggested that the AsA is effective in the treatment of sepsis (Syed et al. 2014; Marik et al. 2017; Marik 2018) Sepsis condition developed in acutely ill patients that causes a very low or undetectable amount of AsA in the serum (Marik 2018). A low amount of AsA in blood was observed due to a higher rate of oxidation, low level of absorption and elevated loss during urination (Marik 2018). Sepsis also induces the process of glomerular hyperfiltration that causes the reduced absorption of AsA (Armour et al. 2001). It had been reported that administration of 100 mg/kg of AsA on the CLP model of sepsis reduces the elevation of lipid peroxidase, aminotransferase and histamine (Kim and Lee 2004; Marik 2018). Administration of AsA during sepsis also decreases the level of nuclear factor kappa B (NF-kB) and high mobility group box 1 (HMGB1). HMGB1 is an important factor for late pro-inflammatory cytokine (Cárcamo et al. 2002). AsA increases the function of leukocytes, production of lysosomal enzymes, phagocytosis expression of antibody response as well as interferon production (Fig. 3). These activities of AsA made it a potent candidate for therapeutic use in COVID-19 treatment (Platto et al. 2020). The rationale to use AsA during COVID-19 treatment is to minimize oxidative stress which mainly occurs due to severe infection, sepsis and various disease states. Acute respiratory distress syndrome (ARDS) and sepsis are reported during severe COVID-19 infection, hence a high dose of AsA helps in upgrading inflammation and vascular injuries in patients. The current status of different phases of clinical trials is summarized in Table 2.
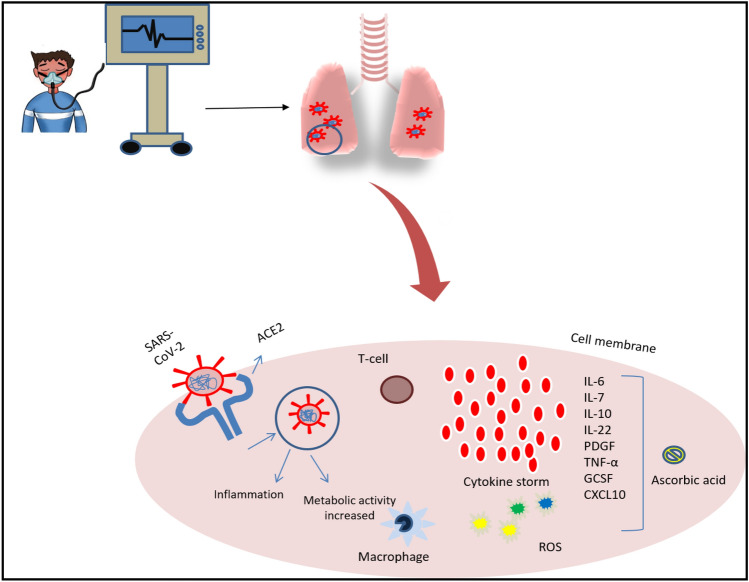
Fig. 3.
During the severe COVID-19 infection, patients experienced cytokine storm [IL-6, IL-7, IL-10, Tumor necrosis factor (TNF α), C Reactive Protein (CRP), granulocyte-colony stimulating factor (GCSF), interferon gamma-induced protein-10 (CXCL10)] and ROS production. A high levels of cytokines storms and ROS production led to inflammation that eventually turn into ARDS and sepsis. These responses induce coagulation and vessel constrictions that cause multi-organ failure. AsA scavenges the free radicles and prevents mitochondria from oxidative stress. It also inhibits the activation of NF-kB thus reducing the pro-inflammatory mediator. Thus, AsA diminishes ROS and cytokines levels and reduces the severity of the disease
Table 2.
Worldwide various ongoing Covid-19 clinical trials with ascorbic acid (adapted and
modified from https://clinicaltrials.gov/api/gui)
| S. No | Experiment Id | Study Objective | Status of experiment | Drugs and dietary supplements | Amount of drugs given/day | Probable outcome | Location |
|---|---|---|---|---|---|---|---|
| 1 | NCT04401150 | Effect of AsA on pneumonia caused by COVID-19 | Phase 3 | AsA | Intravenously ( 50 mg) at every 6 h | Reduction in the mortality rate and PCR levels | Research Center of the CHUS, Sherbrook, Quebec, Canada |
| 2 | NCT04558424 | Effect of AsA on symptoms (cough, fever, Headache, loss of taste and smell, Nausea, Vomiting and diarrhoea) reduction time frame | Phase 1 | Zinc gluconate and AsA | Orally, AsA (1gm) along with Zinc (220 mg) | Reduce the time of symptoms | Bangabandhu Sheikh Mujib Medical University Dhaka, Bangladesh |
| 3 | NCT04395768 | Effect of drug combination on the severity of COVID-19 disease | Phase 2 | Hydroxychloroquine, azithromycin, zinc citrate, AsA, vitamin D3, vitamin B12 | Intravenous, AsA (28 gm), Hydroxychloroquine (200 mg), Azithromycin (250 mg), Zinc Citrate (30 mg), Vitamin D3 (5,000 iu), Vitamin B12 (Methylcobalamin) (500 mcg) | Reduction in the mortality rate, hospital stay and requirement for ventilation | National Institute of Integrative Medicine, Australia |
| 4 | NCT04344184 | Effect of AsA on severity and hypoxemia | Phase 2 | Placebo, AsA | Intravenous, AsA (50 mg/kg) | Reduction in severity and hypoxemic condition in patients | Hunter Holmes McGuire VA Medical Center and Virginia Commonwealth University, Richmond, Richmond, Virginia, United States |
| 5 | NCT04279197 | Effect of these drugs on pulmonary fibrosis after COVID-19 infection | Phase 2 | Fuzheng huayu tablet, AsA, placebo, | Fuzheng Huayu Tablet (4.8 g), AsA (600 mg) | Faster improvement in pulmonary fibrosis | Shuguang Hospital Shanghai, Shanghai, China |
The outcome of the running clinical trial studies of AsA usage in COVID-19 patients may take a long time, hence the administration of AsA can be used as a booster for natural immunity. This approach could be helpful in the fast recovery of COVID-19 patients. Further, the direct consumption of plant products enriched with AsA can be one of the possible ways for the COVID-19 clinical management program, especially for persons who have low immunity.
Bioaccessibility of AsA after digestion
Antioxidant compounds such as AsA possess a highly complex behavior after their consumption. When they travel through the digestive tract, they may undergo many changes that could hamper their distribution, absorption, metabolism, and hence bioaccessibility. AsA is considered an effective antioxidant that protects the cellular components from the damage caused by free radicles. Unlike many low molecular weight compounds, AsA mounts a characteristic behavior during digestion. AsA is mainly released during gastric digestion, which could be assigned to a range of factors in the tract like enzymes, pH, and temperature in the gastrointestinal tract (Sollano-Mendieta et al. 2021). The pharmacokinetics of AsA is mainly regulated by a family of saturable sodium-dependent vitamin C transporters (SVCTs) (Tsukaguchi et al. 1999). SVCTs are usually involved in co-transporting the ascorbate and sodium ions across the membranes resulting in the formation of a concentration gradient (Wilson 2005). The subtypes of these transporters and tissue-specific expression patterns of them lead to a sectionalized distribution of AsA in different organs with a wide range of concentrations. Homeostasis of AsA is affected by various factors, including environment, genetic polymorphism, lifestyle, and diseases. AsA is usually present in the range of 0.2 mM in the heart and muscle, while up to 10 mM in the adrenal gland and brain (Lindblad et al. 2013; Michels et al. 2013). The pharmacology of AsA is also influenced by metabolism. It enters into a recycling process where ascorbate is oxidized into ascorbyl radicals and subsequently experiences dismutation to form ascorbate and dehydroascorbic acid, and finally reduced back to ascorbate. However, this recycling process is failed among smokers and during disease, necessitating a higher intake of AsA to achieve homeostasis (Lykkesfeldt and Tveden-Nyborg 2019).
Strategies to increase AsA in plants
Biofortification of AsA in plants has many positive effects like it is reported to increase the postharvest shelf-life of fruits, provide resistance against various pathogens and high amount of AsA also improves human health. Various approaches have been used for the biofortification of AsA which are discussed below.
Manipulation of the SW pathway
The SW pathway is reported as one of the predominant pathways for the biosynthesis of AsA in different plants. Previously, transgenic approaches have been used for the biofortification of AsA in various plants. Different genes of the SW pathway have been overexpressed in plants to see their role in increasing AsA content (Locato et al. 2013; Macknight et al. 2017). It is manifested that overexpression of the GGP gene can act as a potential candidate to increase the AsA content in plants (Macknight et al. 2017). It was also reported that GMP and GGP could synergistically control the biosynthesis of AsA (Macknight et al. 2017; Paciolla et al. 2019). In Arabidopsis, overexpression of individual genes such as PMI, GMP, GME and GPP did not show a positive effect on the enhancement of AsA. While overexpression of the GGP alone in Arabidopsis showed 2.5-fold high ascorbate content as compared to control plants (Yoshimura et al. 2014). Overexpression of Arabidopsis derived GGP in rice has shown 2.5-fold high AsA (Ali et al. 2019). A kiwi (Actinidia chinensis) derived gene coding for GGP (AcGGP) overexpressed in tomato, potato and strawberry showed 6-fold, 3-fold and 2-fold enhanced AsA, respectively (Bulley et al. 2012). However, overexpression of GGP in tomatoes has led to some morphological fruit alterations, such as fewer numbers of seed formation (Bulley et al. 2012; Macknight et al. 2017). When Acerola (a well-known crop with a high content of AsA) derived GGP gene was expressed under the control of leaf specific promoter in rice it showed a 2.5-fold high content of AsA in the foliar tissue of transgenic rice, without any significant changes in morphology and also confront multi-stress tolerance (Ali et al. 2019). Interestingly, it was also seen that genome editing of an upstream open reading frame (uORF) that is a negative regulator of GGP2, increased the AsA content by 150% in lettuce and provided the tolerance against oxidative stress (Zhang et al. 2018). Transient expression of Triticum aestivum derived GGP (TaGGP) in tobacco increased the ascorbate level upto 5-fold in leaf tissue and revealed the important rate-limiting role of GGP in ascorbate biosynthesis in plants (Broad et al. 2019). A recent study on rice showed that overexpression of rice GGP (OsGGP) increases the AsA 5-fold in foliar leaves than in wild type and its expression also increases the bioavailability of iron (Broad et al. 2020). Three GGP paralogs are reported in the apple and the study showed that the GGP1 allele plays an important role in the regulation of AsA in apples (Mellidou et al. 2012). This study gave a clue that a single-nucleotide polymorphism in GGP allele could be a potent candidate for breeding to improve AsA levels in fruit crops (Paciolla et al. 2019). The multigenic approach can be an excellent alternative strategy to obtain a high level of AsA in crops. The co-expression of kiwi derived GME and GGP in Arabidopsis revealed 7-fold enhanced content of AsA (Bulley et al. 2009). In Arabidopsis, overexpression of GGP alone has increased 2.9-fold of AsA, while co-expression of GGP-GPP and GGP-GLDH elevated AsA up to 4.1-fold (Zhou et al. 2012). Overexpression of Acerola derived GGP, GMP and GME in tomato protoplasts caused a 4-fold higher accumulation of AsA than the wild type (Suekawa et al. 2019). Stable transformation and pyramiding of GME, GMP, GGP and GPP in tomatoes showed the elevated amount of AsA in leaves (2-fold) and fruits (0.4-fold) (Li et al. 2019). Furthermore, these lines showed better AsA transport capability along with fruit shape and size (Li et al. 2019).
Investigation of other AsA biosynthetic pathway genes
The overexpression of genes derived from the other alternative pathways has also demonstrated an affirmative effect on AsA biofortification in crops. Transgenic lines of potato overexpression of rat derived GulLO had shown more AsA accumulation and better abiotic stress tolerance (Hemavathi et al. 2010). Overexpression of Arabidopsis GulLO (AtGulLO) in tobacco increases the 3-fold AsA than control plants (Maruta et al. 2010). Moreover, in Arabidopsis, overexpression of the same AtGulLO gene leads to increased vitamin C along with better growth and improved biomass of shoots and roots with tolerance to abiotic stress (Lisko et al. 2013).
Exciting results were attained by manipulating the galacturonate pathway. Overexpression of strawberry (Fragaria ananasa) derived GalUR (FaGalUR) in potato has enhanced 2-fold higher vitamin C than wild type (Hemavathi et al. 2010). It also provides the plant with a higher tolerance for abiotic stress. Another study in tomatoes suggested that pectin methylesterase, polygalacturonase, and UDP-D-glucuronic-acid-4-epimerase are components of the D-galacturonate pathway. Inhibition of pectin methylesterase reduced the AsA content (Rigano et al. 2018).
Agronomic practices to enhance the AsA content
Agronomic approaches are being used to enhance the AsA content in various crops. Irrigation, fertilizers, and salinity level substantially influence the AsA content in plants (Lee and Kader 2000). Irrigation is an important factor for the proper growth and development of plants. Studies showed that the AsA content of plants could be increased by less frequent irrigation (Lee and Kader 2000). Hot pepper (Capsicum annuum cv. Battle) showed a 23% higher AsA content in less irrigated crops than in the high irrigated conditions (Ahmed et al. 2014). A similar type of result was also noticed in leek (Allium porrum), where less frequent irrigation increases the AsA content along with dietary fiber, and other micronutrients (Lee and Kader 2000). Another aspect of increasing the AsA content in crops is by irrigation of plants with moderate saline water. Increasing the salinity level from 3 to 6 dS/m in hydroponics induces the AsA level along with α-tocopherol and dry matters in tomato fruits. In Italy, saline irrigation has been performed to enhance the flavor of tomatoes and other vegetables (Locato et al. 2013). Similarly, optimum use of fertilizers is essential for the better growth and development of plants. However, the excessive use of nitrogen has lowered the sugars, fibers, and AsA contents in citrus and cucumber fruits (Zhang et al. 2016; Liao et al. 2019). Therefore, the content of AsA can be increased in plants by less frequent irrigation and providing low nitrogen. AsA content can also be increased by the application of methyl jasmonate and gibberellic acid. Methyl jasmonate is involved in stress signaling pathways and its treatment augments the AsA content 2-fold (Locato et al. 2013; Léchaudel et al. 2018). Gibberellic acid is a primary hormone and has a significant role in plant growth and development. It was reported that the treatment of pineapple and strawberries fruits with 50 and 75 mg/L gibberellic acid, respectively increases the AsA content up to 10.5% in comparison to control fruits (Léchaudel et al. 2018; Taghavi et al. 2019).
The selection of appropriate genotype of plant species could also impact the higher accumulation of AsA. The genotypic study showed that varieties of the same crop showed different levels of AsA (Magwaza et al. 2017). Such variability of AsA was reported in the citrus fruits, potato, and maize crops (Locato et al. 2013; Magwaza et al. 2017). For instance, commercial citrus fruit cultivars such as mandarin (Citrus unshiu Marc.) and orange (Citrus sinensis Osb.) showed significant differences in AsA content. Different cultivars of mandarins contain 18.2 to 31.6 mg/100 g AsA, while oranges have deviated AsA content from 18.2 to 22.2 mg/100 g (Magwaza et al. 2017). Several experiments regarding AsA content in potato tuber (Solanum tuberosum) have been thoroughly studied. Potato is the highest grown tuber worldwide and an important food source in several developing and developed countries. A study conducted by the North American potato breeding program on 75 genotypes has revealed that contrasting genotypes produce different amounts of AsA in response to the same environmental conditions (Locato et al. 2013). A similar type of study was conducted in Italy by considering 20 varieties of potato and noted variations in AsA levels. Hence, these studies showed that the selection of AsA enriched variety could be considered a good agriculture practice.
Effect of processing and storage on the bioavailability of AsA
Vitamins are an essential part of a diet, and their deficiency can lead to hypovitaminosis or avitaminosis in many cases. Other than their bioavailability in vegetables and fruits, the processing of these sources is also a critical factor that could affect the stability of these biomolecules and hence the bioaccessibility. It necessitates monitoring and maintaining the conditions during the processing and storage of vegetables and fruits (Giannakourou and Taoukis 2021). In contrast to other vitamins, AsA is less stable and easily degraded during processing. It is relatively labile and prone to different means of degradation during processing leading to nutritional loss. Due to its fluctuating behavior, it is used to indicate nutrient quality deterioration during processing and post-processing handling and storage (Giannakourou and Taoukis 2021). Generally, the processing of foods involves their exposure to aeration, high temperatures, oxygen, and light which might result in modification of composition in processed vegetables and fruits associated with some adverse effects (Gamboa-Santos et al. 2013). During processing, these factors and conditions stimulate the oxidation of L-ascorbic acid to L-dehydroascorbic acid, later being very unstable (Cruz et al. 2008). Subsequently, due to the hydrolysis and lactone ring-opening, L-dehydroascorbic acid gets converted into an inactive form of vitamin called 2, 3-diketogulonic acid (Leong and Oey 2012). AsA is associated with low-temperature stability and oxidizes very quickly. Hence, the processing at elevated temperatures could cause the deterioration of AsA in the processed material compared to the fresh one. Depending upon the temperature range, duration, and oxygen exposure during processing, this deterioration can vary from 20–90%. The extent of retention and stability of AsA is found to be associated with the retention level of other vitamins; therefore, it is very crucial to pore over various food processing parameters and the extent to which it is pernicious for the quality of the final product (Uddin et al. 2002; Marfil et al. 2008; Albahrani and Greaves 2016). Conventional food processing methods like steaming, cooking, boiling, and microwaves also lead to differential degradation of AsA in food where the maximum loss of AsA was observed under microwaves, and the least was in steaming. The boiling of foodstuffs also resulted in a 27–69% loss of AsA in potatoes, carrots, pepper, cabbage, eggplant, and cauliflower (Tincheva 2019). Therefore, it is required to consider the type of raw material and an attentive adjustment of various processing parameters before processing to avert the excessive losses of AsA.
Different studies showed the application of several non-thermal food processing techniques for enhancing their nutritional values and storage. Further, the development of novel bioprocessing techniques will not only subsidize safer but health-promoting and nutritious food leading to the high bioavailability of various biomolecules, including AsA (Mieszczakowska-Frąc et al. 2021). Freezing technology has proven to be the best method for preserving AsA in vegetables and fruits. Quick freezing of fresh material after harvesting help in the retention of AsA. The pasteurization and pressing of material during processing resulted in a considerable loss of AsA in strawberry juices (Klopotek et al. 2005). To eliminate this loss, a new method called high-temperature short time (HTST) has been proposed, where the aseptic conditions are used for placing the aseptic package under high temperature for a short span of time. However, using the HTST method might lead to some unwanted changes in the aroma and color of the product (Koutchma et al. 2016). Another technique for fruitful processing of food material is freeze-drying, which could be used as an alternative to convective drying under hot air (Ali et al. 2016). Processing of different foodstuffs like cranberries, sour cherries, strawberries, and blueberries through freeze-drying has resulted in high AsA retention compared to other conventional methods (Nemzer et al. 2018).
Conclusions
Ascorbic acid (vitamin C) plays a prime role in human health and physiology, and its deficiency can lead to many diseases. It is also essential for several enzyme activities. Vitamin C ameliorates the immune response against various diseases and several reports suggested that it could be a good candidate for reducing the negative effect of several diseases such as skin disorders, cancer and COVID-19. Recommended dietary allowance (RDA) for AsA is 75 to 90 mg/day but major cereals and food crops contain a very low amount of AsA. Therefore, the enhancement of AsA in these crops by biofortification and good agronomical practices could be considered aspromising approaches. While considering the fact that AsA is extremely sensitive to storage conditions and its limited bioavailability and bioaccessibility after digestion led the foundation to enhance AsA in crop plants. In addition, AsA is also essential for plant growth and development due to its involvement in signaling and hormone synthesis. AsA helps plants to cope with various biotic and abiotic stresses and also plays role in defense response against different pathogens in plants.
Acknowledgements
The authors express their gratitude to the National Agri-Food Biotechnology Institute (NABI) for research facilities and the Department of Biotechnology (DBT), Government of India for the grant (BT/PR25789/GET/119/97/2017). The present research was also supported by the Biotechnology Industry Research Assistance Council (BIRAC) for a banana biofortification project grant. SC is thankful to Panjab University Chandigarh and SK is thankful to the Central University of Punjab for PhD registration. Authors would like to acknowledge DBT-eLibrary Consortium (Del-CON) for providing access to online journals.
Author contributions
S.T. conceived and designed the research. S.C. performed the literature survey. S.C., S.K. and S.T. wrote the manuscript. R.K.B and K.K. contributed to the editing and revision of the manuscript.
Funding
This work was supported by the Department of Biotechnology (DBT), Ministry of Science & Technology, Government of India.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that they have no conflicts of interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed AF, Yu H, Yang X, Jiang W. Deficit irrigation affects growth, yield, vitamin C content, and irrigation water use efficiency of hot pepper grown in soilless culture. HortScience. 2014;49:722–728. doi: 10.21273/hortsci.49.6.722. [DOI] [Google Scholar]
- Akram NA, Shafiq F, Ashraf M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albahrani AA, Greaves RF. Fat-soluble vitamins: clinical indications and current challenges for chromatographic measurement. Clin Biochem Rev. 2016;37:27–47. [PMC free article] [PubMed] [Google Scholar]
- Ali B, Pantha S, Acharya R, et al. Enhanced ascorbate level improves multi-stress tolerance in a widely grown indica rice variety without compromising its agronomic characteristics. J Plant Physiol. 2019;240:152998. doi: 10.1016/j.jplph.2019.152998. [DOI] [PubMed] [Google Scholar]
- Ali MA, Yusof YA, Chin NL, Ibrahim MN (2016) Effect of different drying treatments on colour quality and ascorbic acid concentration of guava fruit. Int Food Res J. 23
- Alimohammadi M, De Silva K, Ballu C, et al. Reduction of inositol (1,4,5)-trisphosphate affects the overall phosphoinositol pathway and leads to modifications in light signalling and secondary metabolism in tomato plants. J Exp Bot. 2012;63:825–835. doi: 10.1093/jxb/err306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KE. Porphyria cutanea tarda: a possible role for ascorbic acid. Hepatology. 2007;45:6–8. doi: 10.1002/hep.21514. [DOI] [PubMed] [Google Scholar]
- Armour J, Tyml K, Lidington D, Wilson JX. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol. 2001;90:795–803. doi: 10.1152/jappl.2001.90.3.795. [DOI] [PubMed] [Google Scholar]
- Awad J, Stotz HU, Fekete A, et al. 2-Cysteine Peroxiredoxins and Thylakoid Ascorbate Peroxidase create awater-water cycle that is essential to protect the photosynthetic apparatus under high light stress conditions. Plant Physiol. 2015;167:1592–1603. doi: 10.1104/pp.114.255356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Qu X, Jiang X, et al. Journal of cancer association between dietary vitamin C Intake and risk of prostate cancer : a meta-analysis involving 103, 658 subjects. Journal of Cancer. 2015 doi: 10.7150/jca.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini E, De Tullio MC. Ascorbic acid and ozone: novel perspectives to explain an elusive relationship. Plants. 2019;8:122. doi: 10.3390/plants8050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos GMJ. The effect of vitamin C (Ascorbic Acid ) in the treatment of patients with cancer : a systematic review. Nutrients. 2019;11(5):977. doi: 10.3390/nu11050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botanga CJ, Bethke G, Chen Z, et al. Metabolite profiling of Arabidopsis inoculated with Alternaria brassicicola reveals that ascorbate reduces disease severity. Mol Plant-Microbe Interact. 2012;25:1628–1638. doi: 10.1094/MPMI-07-12-0179-R. [DOI] [PubMed] [Google Scholar]
- Boubakri H (2017) The role of ascorbic acid in plant–pathogen interactions. In: Ascorbic acid in plant growth, development and stress tolerance. Springer, pp 255–271
- Broad RC, Bonneau JP, Beasley JT, et al. Genome-wide identification and characterization of the GDP-L-galactose phosphorylase gene family in bread wheat. BMC Plant Biol. 2019;19:1–15. doi: 10.1186/s12870-019-2123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad RC, Bonneau JP, Beasley JT, et al. Effect of rice GDP-L-galactose phosphorylase constitutive overexpression on ascorbate concentration, stress tolerance, and iron bioavailability in rice. Front Plant Sci. 2020 doi: 10.3389/fpls.2020.595439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley S, Laing W. The regulation of ascorbate biosynthesis. Curr Opin Plant Biol. 2016;33:15–22. doi: 10.1016/j.pbi.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Bulley SM, Rassam M, Hoser D, et al. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot. 2009;60:765–778. doi: 10.1093/jxb/ern327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley S, Wright M, Rommens C, et al. Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol J. 2012;10:390–397. doi: 10.1111/j.1467-7652.2011.00668.x. [DOI] [PubMed] [Google Scholar]
- Cárcamo JM, Pedraza A, Bórquez-Ojeda O, Golde DW. Vitamin C suppresses TNFα-induced NFκB activation by inhibiting IκBα phosphorylation. Biochemistry. 2002;41:12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- Chambial S, Dwivedi S, Shukla KK, et al. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013;28:314–328. doi: 10.1007/s12291-013-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S, Chaudhary R, Tiwari S. Contribution of crop biofortification in mitigating vitamin deficiency globally. Genome Eng Crop Improv. 2021 doi: 10.1002/9781119672425.ch7. [DOI] [Google Scholar]
- Conklin PL, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant, Cell Environ. 2004;27:959–970. doi: 10.1111/j.1365-3040.2004.01203.x. [DOI] [Google Scholar]
- Cruz RMS, Vieira MC, Silva CLM. Effect of heat and thermosonication treatments on watercress (Nasturtium officinale) vitamin C degradation kinetics. Innov Food Sci Emerg Technol. 2008;9:483–488. doi: 10.1016/j.ifset.2007.10.005. [DOI] [Google Scholar]
- da Costa Daniele TM, de Bruin PFC, de Matos RS, et al. Exercise effects on brain and behavior in healthy mice, Alzheimer’s disease and Parkinson’s disease model—a systematic review and meta-analysis. Behav Brain Res. 2020;383:112488. doi: 10.1016/j.bbr.2020.112488. [DOI] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, et al. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- Drisko JA, Serrano OK, Spruce LR, et al. Treatment of pancreatic cancer with intravenous vitamin C: a case report. Anticancer drugs. 2018;29:373–379. doi: 10.1097/CAD.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MJ, Wang ZY, Jones MA, et al. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci U S A. 2007;104:11772–11777. doi: 10.1073/pnas.0700574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres S, Tenhaken R. Myoinositol oxygenase controls the level of myoinositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiol. 2009;149:1042–1049. doi: 10.1104/pp.108.130948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Gamboa-Santos J, Soria AC, Pérez-Mateos M, et al. Vitamin C content and sensorial properties of dehydrated carrots blanched conventionally or by ultrasound. Food Chem. 2013;136:782–788. doi: 10.1016/j.foodchem.2012.07.122. [DOI] [PubMed] [Google Scholar]
- Giannakourou MC, Taoukis PS (2021) Effect of alternative preservation steps and storage on vitamin c stability in fruit and vegetable products: critical review and kinetic modelling approaches. Foods. 10.3390/foods10112630 [DOI] [PMC free article] [PubMed]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Golding PH. Experimental folate deficiency in human subjects : what is the influence of vitamin C status on time taken to develop megaloblastic anaemia ? BMC Hemat. 2018 doi: 10.1186/s12878-018-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet L, Ouerdane L, Flis P, et al. Ascorbate efflux as a new strategy for iron reduction and transport in plants. J Biol Chem. 2014;289:2515–2525. doi: 10.1074/jbc.M113.514828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE. A critical review of vitamin C for the prevention of age-related cognitive decline and alzheimer’s disease. J Alzheimer’s Dis. 2012;29:711–726. doi: 10.3233/JAD-2012-111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- Hemavathi UCP, Akula N, et al. Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett. 2010;32:321–330. doi: 10.1007/s10529-009-0140-0. [DOI] [PubMed] [Google Scholar]
- Heyneke E, Luschin-Ebengreuth N, Krajcer I, et al. Dynamic compartment specific changes in glutathione and ascorbate levels in Arabidopsis plants exposed to different light intensities. BMC Plant Biol. 2013 doi: 10.1186/1471-2229-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov Kavkova E, Blöchl C, Tenhaken R. The Myo-inositol pathway does not contribute to ascorbic acid synthesis. Plant Biol. 2019;21:95–102. doi: 10.1111/plb.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiddle G, Pastori GM, Bernard S, et al. Effects of leaf ascorbate content on defense and photosynthesis gene expression in Arabidopsis thaliana. Antioxidants Redox Signal. 2003;5:23–32. doi: 10.1089/152308603321223513. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee SM. Effect of ascorbic acid on hepatic vasoregulatory gene expression during polymicrobial sepsis. Life Sci. 2004 doi: 10.1016/j.lfs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kim J, Kwon J, Noh G, Lee SS. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488–494. doi: 10.4162/nrp.2013.7.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopotek Y, Otto K, Böhm V. Processing strawberries to different products alters contents of vitamin C, total phenolics, total anthocyanins, and antioxidant capacity. J Agric Food Chem. 2005;53:5640–5646. doi: 10.1021/jf047947v. [DOI] [PubMed] [Google Scholar]
- Koutchma T, Popović V, Ros-Polski V, Popielarz A. Effects of ultraviolet light and high-pressure processing on quality and health-related constituents of fresh juice products. Compr Rev Food Sci Food Saf. 2016;15:844–867. doi: 10.1111/1541-4337.12214. [DOI] [PubMed] [Google Scholar]
- Kukongviriyapan U, Pannangpetch P, Kukongviriyapan V, et al. Curcumin protects against cadmium-induced vascular dysfunction, hypertension and tissue cadmium accumulation in mice. Nutrients. 2014;6:1194–1208. doi: 10.3390/nu6031194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léchaudel M, Darnaudery M, Joët T, et al. Genotypic and environmental effects on the level of ascorbic acid, phenolic compounds and related gene expression during pineapple fruit development and ripening. Plant Physiol Biochem. 2018;130:127–138. doi: 10.1016/j.plaphy.2018.06.041. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- Leong SY, Oey I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012;133:1577–1587. doi: 10.1016/j.foodchem.2012.02.052. [DOI] [Google Scholar]
- Li X, Ye J, Munir S, et al. Biosynthetic gene pyramiding leads to ascorbate accumulation with enhanced oxidative stress tolerance in tomato. Int J Mol Sci. 2019;20:1–17. doi: 10.3390/ijms20071558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Dong T, Qiu X, et al. Nitrogen nutrition is a key modulator of the sugar and organic acid content in citrus fruit. PLoS ONE. 2019;14:1–18. doi: 10.1371/journal.pone.0223356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad M, Tveden-Nyborg P, Lykkesfeldt J. Regulation of vitamin C homeostasis during deficiency. Nutrients. 2013;5:2860–2879. doi: 10.3390/nu5082860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko KA, Torres R, Harris RS, et al. Elevating vitamin C content via overexpression of myo-inositol oxygenase and l-gulono-1,4-lactone oxidase in Arabidopsis leads to enhanced biomass and tolerance to abiotic stresses. Vitr Cell Dev Biol - Plant. 2013;49:643–655. doi: 10.1007/s11627-013-9568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locato V, Cimini S, De Gara L. Strategies to increase vitamin C in plants: from plant defense perspective to food biofortification. Front Plant Sci. 2013;4:1–12. doi: 10.3389/fpls.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence A, Chevone BI, Mendes P, Nessler CL. myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004;134:1200–1205. doi: 10.1104/pp.103.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Guo X. The Effect of light in vitamin C metabolism regulation and accumulation in mung bean (Vigna radiata) Germination. Plant Foods Hum Nutr. 2020;75:24–29. doi: 10.1007/s11130-019-00787-x. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Tveden-Nyborg P. The pharmacokinetics of vitamin C. Nutrients. 2019 doi: 10.3390/nu5082860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight RC, Laing WA, Bulley SM, et al. Increasing ascorbate levels in crops to enhance human nutrition and plant abiotic stress tolerance. Curr Opin Biotechnol. 2017;44:153–160. doi: 10.1016/j.copbio.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Magrì A, Germano G, Lorenzato A, et al. High-dose Vitamin C enhances cancer immunotherapy. Sci Transl Med. 2020;12:1–13. doi: 10.1126/scitranslmed.aay8707. [DOI] [PubMed] [Google Scholar]
- Magwaza LS, Mditshwa A, Tesfay SZ, Opara UL. An overview of preharvest factors affecting vitamin C content of citrus fruit. Sci Hortic (amsterdam) 2017;216:12–21. doi: 10.1016/j.scienta.2016.12.021. [DOI] [Google Scholar]
- Marfil PHM, Santos EM, Telis VRN. Ascorbic acid degradation kinetics in tomatoes at different drying conditions. LWT-Food Sci Technol. 2008;41:1642–1647. doi: 10.1016/j.lwt.2007.11.003. [DOI] [Google Scholar]
- Marik PE. Hydrocortisone, ascorbic acid and thiamine (HAT therapy) for the treatment of sepsis focus on ascorbic acid. Nutrients. 2018 doi: 10.3390/nu10111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151:1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- Maruta T, Ichikawa Y, Mieda T, et al. The contribution of Arabidopsis homologs of L-gulono-1,4-lactone oxidase to the biosynthesis of ascorbic acid. Biosci Biotechnol Biochem. 2010;74:1494–1497. doi: 10.1271/bbb.100157. [DOI] [PubMed] [Google Scholar]
- Mellidou I, Chagné D, Laing WA, et al. Allelic variation in paralogs of GDP-L-galactose phosphorylase is a major determinant of vitamin C concentrations in apple fruit. Plant Physiol. 2012;160:1613–1629. doi: 10.1104/pp.112.203786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels AJ, Hagen TM, Frei B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu Rev Nutr. 2013;33:45–70. doi: 10.1146/annurev-nutr-071812-161246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieszczakowska-Frąc M, Celejewska K, Płocharski W. Impact of innovative technologies on the content of vitamin C and its bioavailability from processed fruit and vegetable products. Antioxidants. 2021;10:1–19. doi: 10.3390/antiox10010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect Med. 2013;3:1–13. doi: 10.1101/cshperspect.a011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitmesser SH, Ye Q, Evans M, Combs M. Determination of plasma and leukocyte vitamin C concentrations in a randomized, double-blind, placebo-controlled trial with Ester-C®. Springerplus. 2016 doi: 10.1186/s40064-016-2605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A, Manjunath K, Ranjan RK, et al. COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020;16:e1008762. doi: 10.1371/journal.ppat.1008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli MB, Gambardella J, Castellanos V, et al. Vitamin C and cardiovascular disease: an update. Antioxidants. 2020;9:1–23. doi: 10.3390/antiox9121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir S, Mumtaz MA, Ahiakpa JK, et al. Genome-wide analysis of Myo-inositol oxygenase gene family in tomato reveals their involvement in ascorbic acid accumulation. BMC Genomics. 2020;21:1–15. doi: 10.1186/s12864-020-6708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemzer B, Vargas L, Xia X, et al. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018;262:242–250. doi: 10.1016/j.foodchem.2018.04.047. [DOI] [PubMed] [Google Scholar]
- Paciolla C, Fortunato S, Dipierro N, et al. Vitamin C in plants: From functions to biofortification. Antioxidants. 2019 doi: 10.3390/antiox8110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M, Section CN, et al. HHS Public Access. 2017;22:463–493. doi: 10.1111/odi.12446.Vitamin. [DOI] [Google Scholar]
- Panich U, Tangsupa-A-Nan V, Onkoksoong T, et al. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch Pharm Res. 2011;34:811–820. doi: 10.1007/s12272-011-0515-3. [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, et al. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell. 2003;15:939–951. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platto S, Xue T, Carafoli E. COVID19: an announced pandemic. Cell Death Dis. 2020 doi: 10.1038/s41419-020-02995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyby M, Langer M. On the physiological and cellular homeostasis of ascorbate. Cellular & Molecular Biology Letter. 2020;25:32. doi: 10.1186/s11658-020-00223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran RD, Vashist P, Gupta SK, et al. Prevalence and risk factors for vitamin C deficiency in North and South India: a two centre population based study in people aged 60 years and over. PLoS ONE. 2011;6:1–8. doi: 10.1371/journal.pone.0028588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano MM, Lionetti V, Raiola A, et al. Pectic enzymes as potential enhancers of ascorbic acid production through the D-galacturonate pathway in Solanaceae. Plant Sci. 2018;266:55–63. doi: 10.1016/j.plantsci.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Rowe S, Carr AC. Global vitamin c status and prevalence of deficiency: A cause for concern? Nutrients. 2020;12:1–20. doi: 10.3390/nu12072008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga G, Giorgetti A, Fufezan C, et al. Mutation analysis of violaxanthin de-epoxidase identifies substrate-binding sites and residues involved in catalysis. J Biol Chem. 2010;285:23763–23770. doi: 10.1074/jbc.M110.115097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy N, Creagan E, Witzig T, Levine M. Ascorbic acid in cancer treatment: let the phoenix fly. Cancer Cell. 2018;34:700–706. doi: 10.1016/j.ccell.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. Ascorbic acid metabolism and functions: a comparison of plants and mammals. Free Radic Biol Med. 2018;122:116–129. doi: 10.1016/j.freeradbiomed.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollano-Mendieta XC, Meza-Márquez OG, Osorio-Revilla G, et al. Effect of in vitro digestion on the antioxidant compounds and antioxidant capacity of 12 plum (Spondias purpurea l.) ecotypes. Foods. 2021;10(9):1995. doi: 10.3390/foods10091995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelheart C, Alegre ML, Baldet P, et al. The effect of low ascorbic acid content on tomato fruit ripening. Planta. 2020 doi: 10.1007/s00425-020-03440-z. [DOI] [PubMed] [Google Scholar]
- Stojkovic-Filipovic J, Kittler H. Dermatoscopy of amelanotic and hypomelanotic melanoma. JDDG J Der Dtsch Dermatologischen Gesellschaft. 2014;12:467–472. doi: 10.1111/ddg.12368. [DOI] [PubMed] [Google Scholar]
- Suekawa M, Fujikawa Y, Inoue A, et al. High levels of expression of multiple enzymes in the Smirnoff-Wheeler pathway are important for high accumulation of ascorbic acid in acerola fruits. Biosci Biotechnol Biochem. 2019;83:1713–1716. doi: 10.1080/09168451.2019.1608808. [DOI] [PubMed] [Google Scholar]
- Syed AA, Knowlson S, Sculthorpe R, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014 doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi T, Siddiqui RK, Rutto L. The Effect of preharvest factors on fruit and nutritional quality in strawberry. Strawb - Pre- Post-Harvest Manag Tech High Fruit Qual. 2019 doi: 10.5772/intechopen.84619. [DOI] [Google Scholar]
- Tincheva PA. The effect of heating on the vitamin C content of selected vegetables. World J Adv Res Rev. 2019;3:27–32. doi: 10.30574/wjarr.2019.3.3.0073. [DOI] [Google Scholar]
- Tollefson MM, Bruckner AL, Cohen BA, et al. Atopic dermatitis: skin-directed management. Pediatrics. 2014 doi: 10.1542/peds.2014-2812. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Tokui T, Mackenzie B, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999 doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Behne M, Quiec D, et al. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J Invest Dermatol. 2001;117:1307–1313. doi: 10.1046/j.0022-202x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- Uddin MS, Hawlader MNA, Ding L, Mujumdar AS. Degradation of ascorbic acid in dried guava during storage. J Food Eng. 2002;51:21–26. doi: 10.1016/S0260-8774(01)00031-0. [DOI] [Google Scholar]
- Ueta E, Tadokoro Y, Yamamoto T, et al. The effect of cigarette smoke exposure and ascorbic acid intake on gene expression of antioxidant enzymes and other related enzymes in the livers and lungs of Shionogi rats with osteogenic disorders. Toxicol Sci. 2003;73:339–347. doi: 10.1093/toxsci/kfg082. [DOI] [PubMed] [Google Scholar]
- Umapathy A, Donaldson P, Lim J. Antioxidant delivery pathways in the anterior eye. BioMed Res Int. 2013;2013:207250. doi: 10.1155/2013/207250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser DY, Jansen NJ, Ijland MM, et al. Intracranial bleeding due to vitamin K deficiency: advantages of using a pediatric intensive care registry. Intensive Care Med. 2011;37:1014–1020. doi: 10.1007/s00134-011-2175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005;25:125–25. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Van Montagu M. GDP-Mannose 3′,5′-Epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem. 2003;278:47483–47490. doi: 10.1074/jbc.M309135200. [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Mieda T, Rapolu M, et al. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot. 2007;58:2661–2671. doi: 10.1093/jxb/erm124. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Nakane T, Kume S, et al. Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Biosci Biotechnol Biochem. 2014;78:60–66. doi: 10.1080/09168451.2014.877831. [DOI] [PubMed] [Google Scholar]
- Yussif N, Koranyb N, Abbassc M (2017) Evidence of the effect of intraepidermic vitamin c injection on melanocytes and keratinocytes in gingival tissues. 10.4172/2161-1122.1000417
- Zhang W, Gruszewski HA, Chevone BI, Nessler CL. An arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol. 2008;146:431–440. doi: 10.1104/pp.107.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yu HJ, Zhang XM, et al. Effect of nitrogen deficiency on ascorbic acid biosynthesis and recycling pathway in cucumber seedlings. Plant Physiol. Biochem. 2016;108:222–230. doi: 10.1016/j.plaphy.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Zhang H, Si X, Ji X, et al. Genome editing of upstream open reading frames enables translational control in plants. Nat Biotechnol. 2018;36:894–900. doi: 10.1038/nbt.4202. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tao QC, Wang ZN, et al. Engineering ascorbic acid biosynthetic pathway in Arabidopsis leaves by single and double gene transformation. Biol Plant. 2012;56:451–457. doi: 10.1007/s10535-012-0119-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.