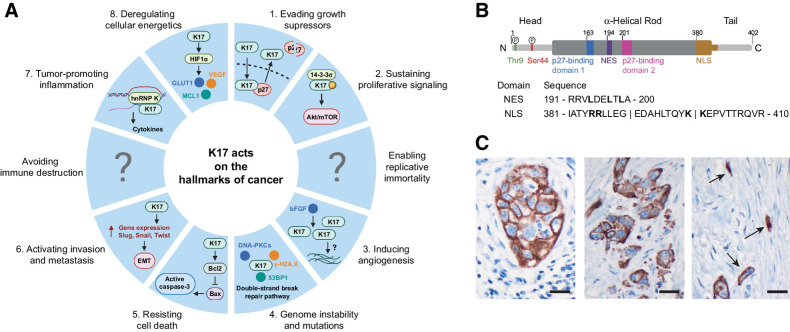
Figure 2.
A, The implication of K17 in each of the 10 deadly hallmarks of cancer. K17 has a function in several hallmarks of cancer. Each piece of the pie chart resembles a hallmark of cancer and represents the mechanism K17 is reported to have in this hallmark, as highlighted in blue. The regions that contain a question mark portray a lack of evidence for K17 in this hallmark and signify that further studies are needed to see whether K17 works mechanistically in cells in this feature. B, Structure of K17. K17 is made up of an α-helical filament domain (residues 84–392) sectioned into four parts of repeated heptads (1A, 1B, 2A, and 2B), and nonhelical head (N terminal; residues 1–83) and tail (C terminal; residues 393–432) domains. K17 has a NES found between residues 191 and 200 of the filament domain and a NLS found between residues 381 and 410 of the protein. It has also been recently reported that there are two phosphorylation sites found on the N-terminal head domain of K17, serine 44 (Ser44) and threonine 9 (Thr9). C, IHC localization of K17 in PDAC. Note diverse patterns of stained tumor cells. Cohesive cluster of large tumor cells (left); smaller tumor cells and apoptotic debris (middle); K17 highlighting small diffusely infiltrative tumor cells, embedded in a densely desmoplastic stroma (right). Original magnification ×600; scale bars, 20 μm.