Figure 4.
RNA elongation inside RdRp of SARS-CoV-2 and human RNA Pol II
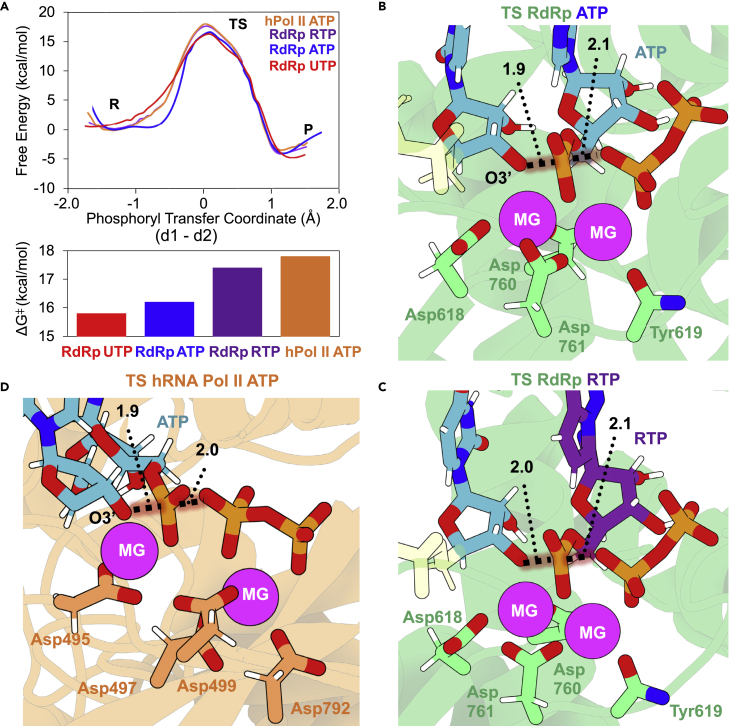
(A) Top, free-energy profiles as a function of the phosphoryl transfer coordinate (d1-d2) for the incorporation of a U, an A, or an R to a nascent viral RNA strand. Profile for the incorporation of the inside human RNA Pol II is also shown. The phosphorylation reaction consists of a nucleophilic attack of the O3′ of the terminal nucleotide on the Pα of the triphosphate nucleotide. Bottom, bar plot displaying free energies of activation for the process for different NTPs and enzymes.
(B and C) Active site views of the TSs were found when ATP (B) and RTP (C) (purple C atoms) are the substrates for the elongation reaction inside RdRp. The phosphoryl group is half-way to being transferred. One Mg2+ activates the O3′ toward nucleophilic attack and stabilizes the negatively charged TS. The other Mg2+ stabilizes the charged TS, as well as the nascent negatively charged PPi molecule. Distances involved in the reaction are shown as dotted lines with their average values in Å.
(D) TS insight of the elongation reaction catalyzed by human RNA pol II.