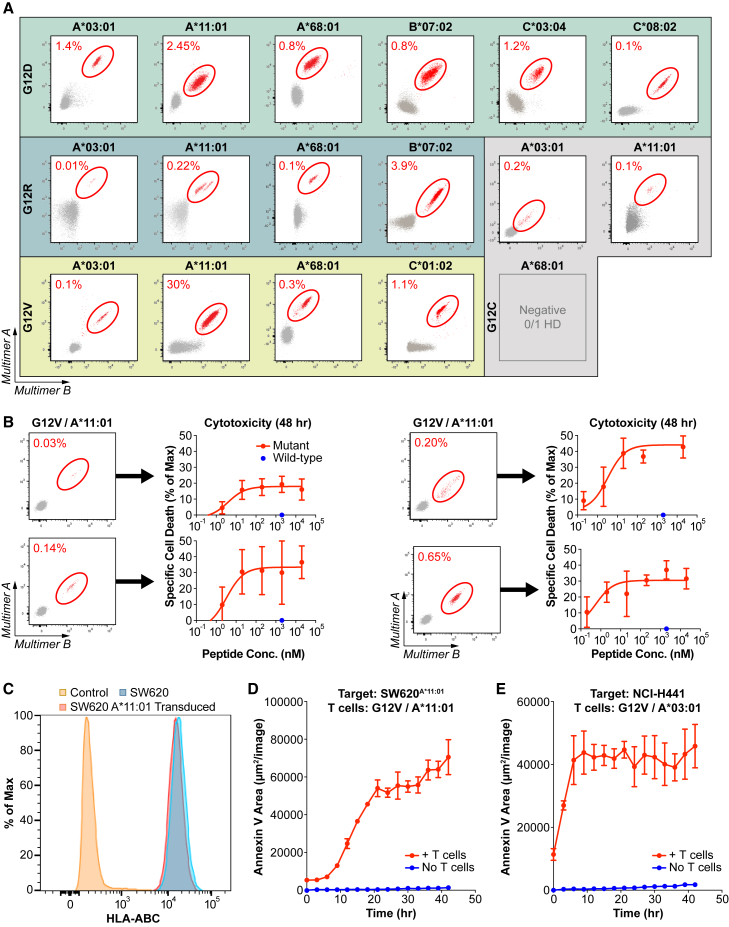
Figure 3.
Evaluation of KRAS neoantigen immunogenicity in healthy donors
(A) Flow cytometry plots of pHLA staining of KRAS neoantigens after naive T cell inductions in healthy donors with the appropriate HLA-I molecules. Multimer-positive populations are circled in red. Naive T cell inductions and multimer analysis were performed for all neoantigens detected by MS, except for KRASG12C on HLA-A∗68:01. See also Figure S3.
(B) Cytotoxicity assay evaluating the specificity of multiple independent T cell cultures stimulated against KRASG12V on HLA-A∗11:01. T cell cultures with identifiable multimer-positive populations (left) were co-cultured with A375 cells transduced to express HLA-A∗11:01 and loaded with increasing amounts of the KRASG12V/HLA-A∗11:01 neoantigens (red dots) or a single concentration of the matching wild-type peptides (blue dots). Data are presented as mean ± SD.
(C–E) Cytotoxicity assay evaluating the ability of a representative T cell culture to recognize endogenously processed and presented KRASG12V neoantigens.
(C) Total HLA-I expression was evaluated on SW620 cells transduced to express HLA-A∗11:01 (red) compared with non-transduced SW620 cells (blue) or isotype control-stained cells (orange). See also Figure S1.
(D and E) Cytotoxicity assays used to evaluate the ability of representative T cell cultures to recognize endogenously processed and presented KRASG12V neoantigens where (D) T cells were co-cultured with SW620A∗11:01 cells and annexin V was measured over time (red) compared with SW620A∗11:01 cells alone (blue) and (E) T cells were co-cultured with NCI-H441 cells, and annexin V was measured over time (red) compared with NCI-H441 cells alone (blue).
Data are presented as mean ± SD and are representative of two independent experiments. See also Figure S4.