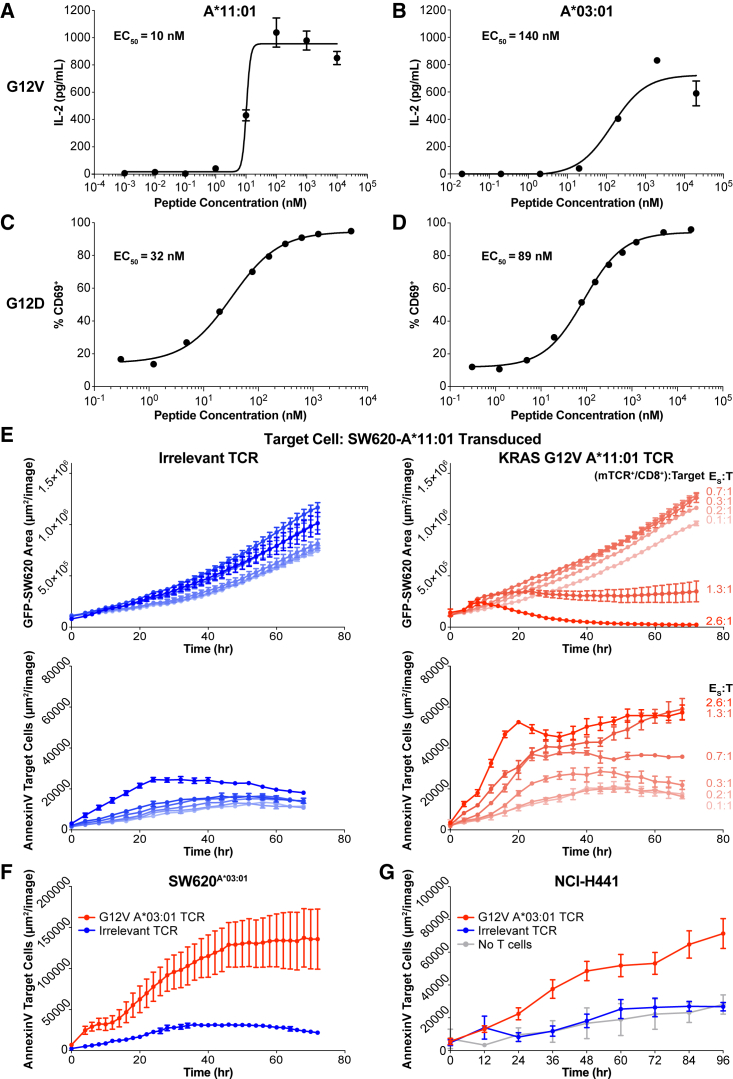
Figure 4.
Characterization of KRAS-specific TCRs on common alleles
(A–D) TCRs isolated from healthy donor T cells stimulated against different KRAS neoantigens were transduced into JurkatCD8 cells. TCR avidity was evaluated by titration of each cognate peptide presented on A375 cells transduced to express the cognate HLA-I molecule. TCR stimulation was assessed by IL-2 secretion or CD69 expression, and half-maximal effective concentration was calculated. (A) TCR specific for KRASG12V on HLA-A∗11:01. (B) TCR specific for KRASG12V on HLA-A∗03:01. (C) TCR specific for KRASG12D on HLA-A∗11:01. (D) TCR specific for KRASG12D on HLA-A∗03:01. Data are presented as mean ± SD and are representative of at least two independent experiments. See also Figure S5.
(E) The TCR from (A) was transduced into PBMCs and used in a cytotoxicity assay against SW620A∗11:01 across a range of specific effector to target (E:T) ratios (red). The ratio was calculated using the number of TCR-transduced CD8+ T cells in the well. As a control, an equivalent number of PBMCs transduced with an irrelevant TCR was co-cultured with SW620A11:01 cells (blue). Both target cell growth (top) and target cell death (bottom) were measured. Data are presented as mean ± SD and are representative of three independent experiments.
(F) The TCR from (B) was transduced into PBMCs and used in a cytotoxicity assay against SW620A∗03:01 (red), and cell death of the target cell was measured. As a negative control, PBMCs transduced with the TCR from (A) were co-cultured with SW620A∗03:01 (blue). Data are presented as mean ± SD and are representative of two independent experiments.
(G) The TCR from (B) was transduced into PBMCs and used in a cytotoxicity assay against NCI-H441 (red), and cell death of the target cell was measured. As a negative control, PBMCs transduced with the TCR from (A) were co-cultured with NCI-H441 (blue). Data are presented as mean ± SD and are representative of two independent experiments.