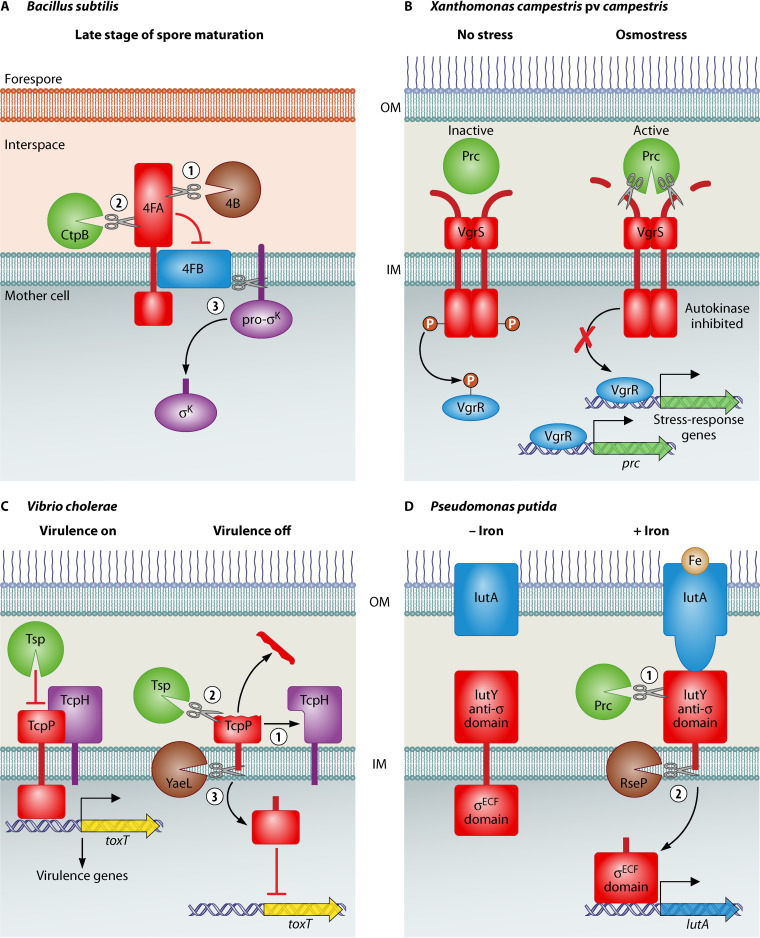
FIG 1.
CTPs involved in regulation. (A) In the final steps of B. subtilis spore development, the SpoIVB protease (4B) removes the C-terminus of SpoIVFA (4FA), which then allows CtpB to remove the 4FA domain that inhibits the SpoIVFB (4FB) protease. 4FB then cleaves pro-sK, releasing active sK into the mother cell cytoplasm. (B) In X. campestris pv. campestris, osmostress activates Prc to cleave the N-terminal periplasmic domain of VgrS. This reduces phosphotransfer to VgrR, which alters its global promoter binding profile, allowing it to induce expression of stress-response genes, and of prc itself, providing a positive feedback loop. (C) In conditions that do not favor V. cholerae virulence gene expression, TcpH dissociates from TcpP, allowing Tsp to cleave close to the C-terminus of TcpP. The truncated TcpP is then further degraded by the YaeL protease. This sequential cleavage of TcpP by Tsp and YaeL inactivates TcpP, so that toxT and the virulence genes controlled by ToxT, are no longer expressed. (D) In P. putida iron-loaded aerobactin (brown circle) binds to the IutA receptor. This causes the IutA signaling domain to interact with IutY, triggering IutY cleavage by Prc. The transmembrane domain of the truncated IutY is then cleaved by RseP (an ortholog of V. cholerae YaeL), which releases the σECF domain into the cytoplasm to induce iutA expression.