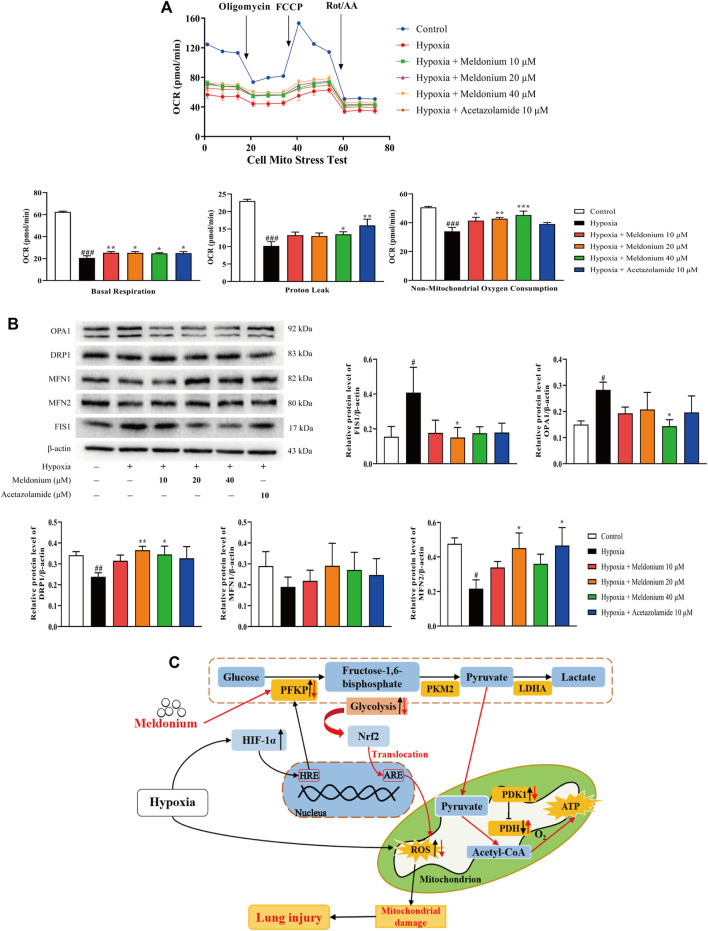
FIGURE 5.
A mechanistic illustration of meldonium amelioration of oxidative stress–induced mitochondrial damage. (A) Oxygen consumption rate (OCR) diagram, basal respiration, proton leak, and non-mitochondrial oxygen consumption in vitro after hypoxia for 24 h were detected using the Cell Mito Stress Test Kit (n > 3). (B) Protein expression of mitochondrial fission and fusion in vitro after hypoxia for 24 h were detected via western blot (n = 3–5). (C) A schematic diagram depicting a potential mechanism by which meldonium regulates glycolysis to alleviate hypoxia-induced lung injury. Mechanistically, meldonium can regulate and interact with PFKP to regulate glycolysis that is one of main energy metabolic pathway, while promoting Nrf2 transfer from the cytoplasm to the nucleus. Substantially, Nrf2 actives downstream pathways to prevent oxidative stress and alleviate mitochondrion damage and homeostasis imbalance, which protect the lung from hypoxia-induced injury. Black arrows denote hypoxia-induced changes. Red arrows denote meldonium-induced changes. ARE, antioxidant response elements; HRE, hypoxia response elements; HIF-1α, hypoxia inducible factor-1α; PFKP, platelet isoform of phosphofructokinase; PKM2, M2 type of pyruvate kinase; LDHA, lactate dehydrogenase A; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; Nrf2, nuclear factor E2-related factor 2; ROS, reactive oxygen species; ATP, adenosine triphosphate. Data are expressed as the mean ± SEM. Statistical analyses were performed using one-way ANOVA followed by Fisher’s LSD test. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the control group; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the hypoxia group.