Abstract
Background
In this post-hoc analysis, data from 2 positive, pivotal, phase 3 trials of esketamine nasal spray (ESK) in treatment-resistant depression (TRD)—short-term study (TRANSFORM-2) and maintenance study (SUSTAIN-1)—were analyzed to evaluate the relationship between dissociation and antidepressant effects of ESK.
Methods
Analysis by responder status, correlation analysis, and mediation analysis were performed to assess the relationships between peak Clinician Administered Dissociative States Scale (CADSS) scores after first (day 1) and last (day 25) ESK dose and change in Montgomery-Åsberg Depression Rating Scale (MADRS) total scores at the first (day 2) and last assessments (day 28) in TRANSFORM-2 and peak CADSS after first maintenance ESK dose and time to relapse in SUSTAIN-1 (only for mediation analysis).
Results
In TRANSFORM-2, the percentage of responders (>50% reduction in MADRS) at day 2 and day 28 did not significantly differ between patients who did vs did not manifest significant dissociation (peak CADSS scores >4 or ≤4, respectively) following the first ESK dose. Spearman correlation coefficients between dissociation and depression improvement were nonsignificant and close to zero. CADSS scores did not significantly mediate the reduction in MADRS at day 2 or 28 in TRANSFORM-2 or the time to depression relapse in SUSTAIN-1. The mean difference in MADRS between ESK and active-control arms persisted beyond day 2 without significant change across time, although the mean peak CADSS scores significantly decreased across consecutive doses and fewer patients experienced significant dissociation after the last ESK dose compared with the first.
Conclusion
Within the dose range tested, the dissociative and antidepressant effects of ESK were not significantly correlated.
Trial registration
NCT02417064 (TRANSFORM-1); NCT02418585(TRANSFORM-2); NCT02493868 (SUSTAIN-1)
Keywords: Antidepressant, dissociation, esketamine, treatment-resistant depression
Significance Statement.
There is disagreement in the literature regarding whether a significant association exists between antidepressant and dissociative effects produced by intravenous ketamine in patients with treatment-resistant depression (TRD). Some studies reported that dissociative effects are linked to the antidepressant efficacy, while others found no evidence for such an association. Using data from phase 3 studies of esketamine nasal spray (ESK) in TRD, we assessed the relationship between antidepressant and dissociative effects. Our findings indicate no significant correlation between the antidepressant efficacy of ESK and either the presence or severity of clinically significant dissociation in short-term (4-week) trials. In a long-term maintenance study followed by randomized withdrawal, the time to depressive relapse was not mediated by dissociation. Furthermore, the peak increase in dissociation diminished over time without any corresponding attenuation of antidepressant response. In conclusion, we did not find any significant correlation between the antidepressant effects and dissociative adverse effects induced by ESK.
Introduction
Intravenous (i.v.) infusions of ketamine and esketamine at subanesthetic doses have demonstrated rapid (within hours) and robust antidepressant effects in patients with treatment-resistant depression (TRD) (Zarate et al., 2006; Ionescu et al., 2019). The clinical improvement in patients with TRD has been observed to persist for several days after a single infusion of ketamine (Zarate et al., 2006; Ionescu et al., 2019).
Among the clinical trials evaluating efficacy and safety of esketamine, Singh et al. demonstrated that the antidepressant effect size of esketamine administered at 0.2 mg/kg i.v. was similar to that of ketamine racemate administered at 0.5 mg/kg i.v. in patients with TRD, consistent with the 2- to 2.5-fold greater potency of esketamine compared with ketamine for N-methyl-D-aspartate receptor (NMDAR) binding (Singh et al., 2016a). Daly et al. demonstrated significant improvement in depressive symptoms following 1 week of treatment with esketamine nasal spray (ESK) administered adjunctively to an oral antidepressant (AD) in patients with TRD (Daly et al., 2018). Subsequently, multiple phase 3 studies have shown meaningful antidepressant effects of ESK following short-term treatment (4 weeks) or maintenance treatment in patients with TRD (Daly et al., 2019; Popova et al., 2019) and in severely ill patients with major depressive disorder (MDD) and active suicidal ideation with intent (Fu et al., 2020; Ionescu et al., 2021). The efficacy of ESK in delaying relapse and its long-term safety and tolerability in TRD have also been demonstrated in 2 phase 3 studies (Daly et al., 2019; Wajs et al., 2020). The US Food and Drug Administration approved ESK nasal spray (SPRAVATO), in conjunction with an oral AD for the treatment of TRD in 2019 (SPRAVATO Prescribing Information 2020), with European Union approval following in 2020. In July 2020, the US Food and Drug Administration also approved SPRAVATO in conjunction with an oral AD for the treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior (Johnson & Johnson, Press Release 2020) with European Union approval for rapid reductions in depressive symptoms in a psychiatric emergency for patients with moderate to severe MDD following in February 2021.
Both antidepressant and dissociative effects have been observed in the clinical dose range of ketamine and esketamine used in previous studies of TRD (Berman et al., 2000; Zarate et al., 2006; Price et al., 2009; Diazgranados et al., 2010a, b; Ballard et al., 2014, 2015; Hu et al., 2016; Ionescu et al., 2019). In studies using racemic ketamine (0.5 mg/kg i.v.) in patients with TRD, findings have been inconsistent regarding whether changes in depression severity correlate with changes in dissociation severity and if the dissociation is causally required for the subsequent antidepressant effects (Ballard and Zarate 2020). Whereas 2 studies found no relationship between these clinical variables (Valentine et al., 2011; Murrough et al., 2013), 2 others reported a significant correlation between the increases in the depersonalization subdomain and overall dissociation measures of the Clinician-Administered Dissociative States Scale (CADSS) (Bremner et al., 1998) and the improvement in depressive symptoms after a single ketamine infusion (Luckenbaugh et al., 2014; Niciu et al., 2018).
Given the inconsistency in the findings reported in the literature (Ballard and Zarate, 2020) and the lack of similar published data for esketamine, the current study examined whether the antidepressant response to ESK treatment is correlated with and/or mediated by dissociative effects. We evaluated the relationship between the antidepressant and dissociative effects of ESK in a post-hoc analysis of 2 phase 3 trials conducted to assess the short-term efficacy of ESK (Fedgchin et al., 2019; Popova et al., 2019) as well as in a post-hoc analysis of a long-term maintenance study that evaluated the efficacy of ESK in sustaining the antidepressant effects in patients with TRD using a randomized withdrawal design (Daly et al., 2019). The relationships between changes in CADSS total score and changes in depression severity based on the Montgomery-Åsberg Depression Rating Scale (MADRS) total score (Montgomery and Asberg 1979) were assessed using correlation analysis, mediation analysis, and analysis by responder status. In addition, the temporal relationships between changes from baseline in the CADSS and MADRS total scores across time were characterized. Notably, compared with the studies that have examined this relationship to date, the sample studied herein was substantially larger in size than the samples assessed in previous studies.
METHODS
Datasets
This post-hoc analysis used data from 3 randomized, double-blind (DB), active-controlled, multicenter, phase 3 studies of ESK: TRANSFORM-1 (short-term, fixed-dose study; NCT02417064), TRANSFORM-2 (short-term, flexible-dose study; NCT02418585), and SUSTAIN-1 (long-term, maintenance study; NCT02493868). Details of the study design and eligibility criteria of these 3 studies were reported elsewhere (Daly et al., 2019; Fedgchin et al., 2019; Popova et al., 2019). Briefly, eligible patients (n = 346) in the TRANSFORM-1 study were randomized to a fixed-dose regimen of either 56 mg ESK, 84 mg ESK, or placebo nasal spray administered with a newly initiated, open-label oral AD (abbreviated ESK [84 mg] + AD, ESK [56 mg] + AD, AD + placebo) (Fedgchin et al., 2019). In the TRANSFORM-2 study, patients (n = 227) were randomized to either a flexible-dose regimen of ESK (56 or 84 mg) + AD or AD + placebo (Popova et al., 2019). In both studies, ESK or placebo nasal spray was administered twice a week during the 4-week DB induction phase (Fedgchin et al., 2019; Popova et al., 2019). In SUSTAIN-1, direct entry patients received flexible dose ESK (56 or 84 mg) twice weekly + AD during the 4-week open-label induction phase. Subsequently, in the 12-week optimization phase, the frequency of ESK dosing was reduced to once weekly for 4 weeks and individualized to weekly or every 2 weeks based on the severity of depressive symptoms. Patients on ESK who achieved stable remission (MADRS total score ≤12 for at least 3 of the last 4 weeks prior to randomization) or stable response (≥50% reduction in the MADRS total score from baseline in each of the last 2 weeks prior to randomization but without achieving remission) at the end of the optimization phase (n = 297) were randomly assigned 1:1 to either continue ESK + AD or switch to placebo nasal spray while continuing the same AD + placebo nasal spray during the variable duration (randomized relapse event-based) maintenance phase (Daly et al., 2019). Dosing of the nasal spray medication during the maintenance phase was individualized to weekly or every other week using a MADRS-based algorithm.
Changes in depression severity were assessed by the change in MADRS total score from baseline over time during the DB treatment induction phase of the TRANSFORM-1 and TRANSFORM-2 studies and throughout the SUSTAIN-1 study (Daly et al., 2019; Fedgchin et al., 2019; Popova et al., 2019). The presence and severity of dissociative symptoms were assessed using the CADSS. The total and component scores were recorded on each ESK treatment day at pre-dose, 40 minutes post-dose, and 1.5 hours post-dose during the DB treatment phase of the TRANSFORM-1 and TRANSFORM-2 studies and during all treatment administration visits of SUSTAIN-1. A CADSS total score >4 was used as the threshold for indicating the presence of clinically meaningful dissociative symptoms based on a previous reported range of CADSS scores in heathy participants (Bremner et al., 1998).
In TRANSFORM-1, the difference between the ESK (84 mg) + AD and AD + placebo treatment groups was not statistically significant for the change from baseline in MADRS total score at day 28 (Fedgchin et al., 2019). Therefore, in accordance with the predefined testing sequence, the ESK (56 mg) + AD treatment group could not be formally evaluated. Notably, the median unbiased estimate (95% confidence interval [CI]) of the treatment difference between the ESK (56 mg) + AD group and the AD + placebo group (−4.1 [−7.67, −0.49]) was comparable with the estimated treatment differences in the other phase 3 studies (Popova et al., 2019; Ochs-Ross et al., 2020). In TRANSFORM-2, treatment with ESK (flexibly dosed between 56 and 84 mg) + AD significantly improved depressive symptoms in patients with TRD (Popova et al., 2019). Since the primary efficacy analysis was significant for both the TRANSFORM-2 study (least squares [LS] mean difference [95% CI]: −4.0 [−7.31, −0.64]; P = .020) and SUSTAIN-1 study (significant delay in relapse among patients achieving stable remission with ESK: hazard ratio [HR] = 0.49; 95% CI = 0.29, 0.84; P = .003 and stable response: HR = 0.30; 95% CI = 0.16, 0.55; P < .001), the post-hoc results from assessments between clinical improvement in depression symptoms and dissociation for these 2 studies are presented in the main body of this manuscript, and the corresponding results for the TRANSFORM-1 study are presented in the online supplement.
The protocols and their respective amendments for all 3 studies were reviewed by an independent ethics committee or institutional review board at each site. The studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. Written informed consent was obtained from all patients before participating in the study.
Statistical Analyses
The analyses presented herein are considered post-hoc. The correlation analysis for TRANSFORM-1 and TRANSFORM-2 studies was based on the post-dose peak changes in CADSS total and component scores, whereas the mediation analysis used post-dose changes in CADSS total scores on the first (day 1) and last (day 25) day of nasal spray treatment and changes from baseline in MADRS total scores at day 2 (the day after the first nasal spray treatment) and day 28 (the day of last MADRS assessment of the induction phase).
Data from SUSTAIN-1 was used only for the mediation analysis based on post-dose changes in CADSS total score on the first day of the DB maintenance phase and time to relapse in those patients who were stable remitters after an initial 16 weeks of treatment with ESK + AD and had proceeded to the randomized withdrawal/maintenance phase.
Mediation Analysis
Causal mediation analysis using a simulation-based approach was performed to examine the mediating role of dissociative side effects on antidepressant effects (Imai et al., 2010) in the TRANSFORM-1 and TRANSFORM-2 studies. An analysis of covariance (ANCOVA) model was used to assess changes from pre-dose in CADSS total scores at 40 minutes post-dose (mediator), with treatment, region (for TRANSFORM-1) or country (for TRANSFORM-2), class of oral antidepressant (serotonin and norepinephrine reuptake inhibitors or selective serotonin reuptake inhibitors) as factors and the baseline MADRS total score as a covariate. The ANCOVA model for changes in MADRS total score from baseline (outcome) included the same factors and covariates in addition to the mediator. In the mediation analysis framework, a direct effect is considered as an independent treatment effect on the outcome that is above and beyond its effect on the mediator, whereas an indirect effect is considered as a treatment effect on the outcome that is accounted for by its effect on the mediator. The direct and indirect effects were estimated by parametric bootstrapping via R-package “mediation.” The proportion of mediated effects is calculated as the ratio of the indirect effect to the sum of the direct and indirect effects. Causal mediation analysis based on the approach of Lange and Hansen (2011) was also performed for the SUSTAIN-1 data. The mediator was change from pre-dose in CADSS total score at 40 minutes post-dose on day 1 of maintenance treatment, and the outcome was the time to relapse. The direct and indirect effects were estimated via R-package “timereg.”
Correlation Analysis
Spearman coefficients along with 95% CIs were computed to assess the relationships between reductions in MADRS total scores and post-dose peak values in the CADSS total and component scores following the first and last nasal spray treatment. Scatter plots were used for graphical representation.
Temporal Analysis
Changes from baseline in MADRS total scores over time expressed using the LS means (±SE) based on the mixed model for repeated measures were graphed (details of the mixed model for repeated measures have been described previously) (Fedgchin et al., 2019; Popova et al., 2019). The peak CADSS scores across time were analyzed in a similar model, and the arithmetic mean (±SE) CADSS score over time was plotted.
RESULTS
Patients and Primary Outcomes of the Trials
Demographic and baseline characteristics and clinical outcomes of the TRANSFORM-1, TRANSFORM-2, and SUSTAIN-1 studies were previously described (Daly et al., 2019; Fedgchin et al., 2019; Popova et al., 2019). In general, the demographic or baseline clinical characteristics were balanced between the ESK + AD and AD + placebo groups in the 2 short-term studies (supplementary Table 1). In SUSTAIN-1, a total of 297 patients with a mean (SD) age of 46.3 (11.13) years, the majority of whom were women (197 [66.3%]), were randomized in the maintenance phase (Daly et al., 2019). The demographics and baseline characteristics were generally comparable between patients who achieved stable response vs stable remission following 16 weeks of treatment with ESK +AD and entered the maintenance phase (supplementary Table 2).
CADSS Scores
Supplementary Table 3 shows mean baseline and peak CADSS total scores in ESK + AD and AD + placebo groups in the TRANSFORM-2 study. In both treatment groups, increases in CADSS score peaked at the 40-minute timepoint (supplementary Table 3 and review of patients’ data at individual level). In the AD + placebo group, the CADSS scores on average were <4 (supplementary Table 3). Moreover, the percentage of patients with a CADSS score >4 was 3.8% (4/104) at day 1 and 4.2% (4/95) at day 25.
Results of Responder Analysis
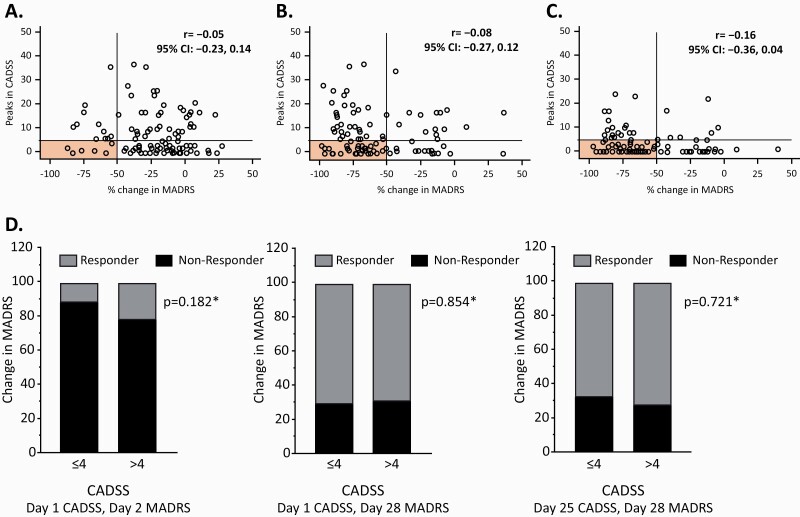
In TRANSFORM-2, after receiving the first ESK dose, 12% of patients manifested an antidepressant response 24 hours after dosing (day 2) without having experienced dissociation (CADSS total score ≤4) acutely after dosing, and 21% of patients showed antidepressant response at day 2 with dissociation observed acutely after dosing (CADSS total score >4) (P = .182) (Figure 1A,D). At day 28 (end of the DB period), 70% of patients had achieved antidepressant response without dissociation having been observed after the initial ESK dose, and 69% showed antidepressant response along with dissociation following the initial ESK dose (P = .854) (Figure 1B,D). Similarly, after the last ESK dose administration (day 25), 68% of patients showed antidepressant response at day 28 without dissociation observed after the final ESK dose (which occurred at day 25), and 71% of patients showed antidepressant response at day 28 with dissociation occurring after the final ESK dose at day 25 (P = .721) at the end of the DB period (day 28) (Figure 1C,D). Thus, the responder rates in the CADSS score ≤4 and >4 groups did not differ significantly at any of the time-points tested. Similar results were observed for the TRANSFORM-1 study (supplementary Figure 1).
Figure 1.
Distribution of participants by dissociative response (assessed by Clinician-Administered Dissociative States Scale [CADSS] score obtained 40 minutes post esketamine administration) and the corresponding antidepressant effect (assessed by change from baseline in Montgomery-Åsberg Depression Rating Scale [MADRS] scores) in TRANSFORM-2. (A–C) Changes in CADSS total scores 40 minutes post esketamine nasal spray (ESK) and corresponding reductions in MADRS total score for each participant (depicted by “O” symbol) for the following correlations: (A) CADSS after first ESK (day 1) and MADRS at day 2: CADSS increase after first ESK treatment session and MADRS change assessed 24 hours later (day 2), n = 109; (B) CADSS after first ESK (day 1) and MADRS at day 28: CADSS after first ESK session and MADRS change at the end of the double blind treatment period (day 28), n = 101; (C) CADSS after last ESK (day 25) and MADRS at day 28: CADSS assessed after last ESK session (day 25) and MADRS change at day 28, n = 101; (D) CADSS scores ≤4 or >4 and antidepressant responders and non-responders: percentage of treatment responders (defined by ≥50% improvement in MADRS total score) and non-responders among patients with CADSS total scores ≤4 vs patients with CADSS total scores >4 for study days indicated on each graph. Shaded areas in graphs A–C correspond to patients who had a response (MADRS) without significant dissociation (CADSS). * Nominal P values (chi-squared test) for proportion of responder patients with and without dissociation. CI, confidence interval; r, Spearman correlation coefficient.
Results of Correlation Analysis
In TRANSFORM-2, the correlation analysis of the relationship between dissociation (increases in CADSS total scores) after first ESK dose at day 1 and the antidepressant response (i.e., reductions in MADRS total scores) at day 2 (r = −0.05 [95% CI = −0.23, 0.14]) and day 28 (r = −0.08 [95% CI = −0.27, 0.12]) showed nonsignificant Spearman correlation coefficients (based on 95% CIs containing 0) close to zero (Table 1). Similarly, no significant correlation was identified between the CADSS total scores following the last ESK dose at day 25 and the depression response at day 28 (r = −0.16 [95% CI = −0.36, 0.04]). A lack of significant correlation was also observed for the corresponding analyses in the TRANSFORM-1 data (supplementary Table 4). Finally, no significant correlation was observed between the improvement in depression severity and the individual subcomponent scores of the CADSS considered separately in the TRANSFORM-2 data (Table 1) and the TRANSFORM-1 data (supplementary Table 4).
Table 1.
Results of Correlation Analysis on the Relationship Between Dissociative Response and the Corresponding Antidepressant Effect in the Transform-2 Cohort
| Correlations with CADSS scores at 40 minutes after first nasal spray (day 1) | ||||||
|---|---|---|---|---|---|---|
| Total | Depersonalization | Derealization | Amnesia | |||
| Change from baseline in MADRS total score | n | r (95% CI) | ||||
| Day 2 | AD + placebo | 99 | −0.05 (−0.25, 0.14) |
0.05 (−0.15, 0.25) |
−0.06 (−0.26, 0.14) |
−0.07 (−0.27, 0.13) |
| ESK + AD | 109 | −0.05 (−0.23, 0.14) |
−0.02 (−0.21, 0.17) |
−0.06 (−0.24, 0.13) |
−0.12 (−0.30, 0.07) |
|
| Day 28 | AD + placebo | 96 | 0.01 (−0.19; 0.21) |
−0.05 (−0.25, 0.15) |
−0.01 (−0.21, 0.19) |
−0.00 (−0.20, 0.20) |
| ESK + AD | 101 | −0.08 (−0.27, 0.12) |
−0.01 (−0.21, 0.18) |
−0.15 (−0.33, 0.05) |
−0.04 (−0.23, 0.16) |
|
| Correlations with CADSS scores at 40 minutes after last nasal spray (day 25) | ||||||
| Day 28 | AD + placebo | 95 | 0.02 (−0.18, 0.22) |
0.04 (−0.16, 0.24) |
−0.13 (−0.32, 0.07) |
0.19 (−0.01, 0.38) |
| ESK + AD | 93 | −0.16(−0.36, 0.04) | 0.00 (−0.20, 0.20) |
−0.19 (−0.38, 0.02) |
−0.20 (−0.39, 0.01) |
|
Abbreviations: AD, antidepressant; CADSS, Clinician-Administered Dissociative States Scale; ESK, esketamine nasal spray; MADRS, Montgomery-Åsberg Depression Rating Scale; r, Spearman correlation coefficient.
Negative r values indicate higher responses on CADSS total scores and more improvements in depression symptoms.
Outcomes of Mediation Analysis
In TRANSFORM-2, the mediation analysis showed insufficient evidence of a mediation effect of dissociation on the antidepressant efficacy after either the first (day 1) or the last (day 25) ESK treatment (based on 95% CIs containing 0) (Table 2). In contrast, the direct effect of ESK on the antidepressant effect was significant: the LS mean difference (95% CI) between treatment groups for the change in MADRS total score that was independent of dissociation effect was −3.62 (−6.62, −0.74) and −4.83 (−8.37, −1.28) after initiation of the first and last ESK dose, respectively.
Table 2.
Mediation Analysis of Changes in CADSS Total Scores on Changes in MADRS Total Scores in TRANSFORM-2 and SUSTAIN-1 Study Cohorts
| Indirect effect | |||||
|---|---|---|---|---|---|
| Mediator | Outcome | Direct effect LS mean (95% Cl) | LS mean (95% Cl) | CI contains 0 | Proportion being mediated |
| TRANSFORM-2 | |||||
| Change in CADSS total score at 40 minutes post-dose (day 1) | Reduction from baseline in MADRS total score (day 2) | −3.62 (−6.62, −0.74) |
−0.45a (−1.85, 0.85) |
Yes | 11% |
| Change in CADSS total score at 40 minutes post-dose (day 25) | Reduction from baseline in MADRS total score (day 28) | −4.83 (−8.37, −1.28) |
−0.42a (−1.95, 0.94) | Yes | 8% |
| SUSTAN-1 | |||||
| Change in CADSS total score at 40 minutes post first maintenance dose | Time to relapse | −2.44 (−4.04, −0.84) |
0.12b (−3.22, 3.45) |
Yes | ~0% |
Abbreviations: CADSS, Clinician-Administered Dissociative States Scale; CI, confidence interval; LS, least squares; MADRS, Montgomery-Åsberg Depression Rating Scale.
Note: The results show no significant effect of dissociation symptoms on mediating the antidepressant effect, but a significant direct effect of esketamine on the antidepressant effect (based on the inclusion and exclusion, respectively, of 0 by the 95% CI).
a The estimated LS mean difference (95% CI) of change in MADRS total score mediated by difference of change in CADSS total score are shown.
b The estimated LS mean difference (95% CI) of change in number of relapses per day per 1000 people mediated by difference of change in CADSS total score are shown.
Similar results were observed in the TRANSFORM-1 study at day 2 (supplementary Table 5). The mediation analysis was not performed at the day 28 timepoint for the TRANSFORM-1 study, however, due to lack of significant difference of antidepressant responses between ESK (84 mg) + AD and AD + placebo groups, as described above (Fedgchin et al., 2019).
Likewise, the mediation analysis of SUSTAIN-1 results also showed a lack of a mediation effect of dissociation on the time to relapse after the first ESK dosing in the maintenance phase (LS mean difference [95% CI] = 0.12 [−3.22, 3.45]), whereas the direct effect of ESK was significant (LS mean difference [95% CI] = −2.44 [−4.04, −0.84]) (Table 2).
Temporal Profiles of Dissociation and Antidepressant Responses Over Repeated Dosing
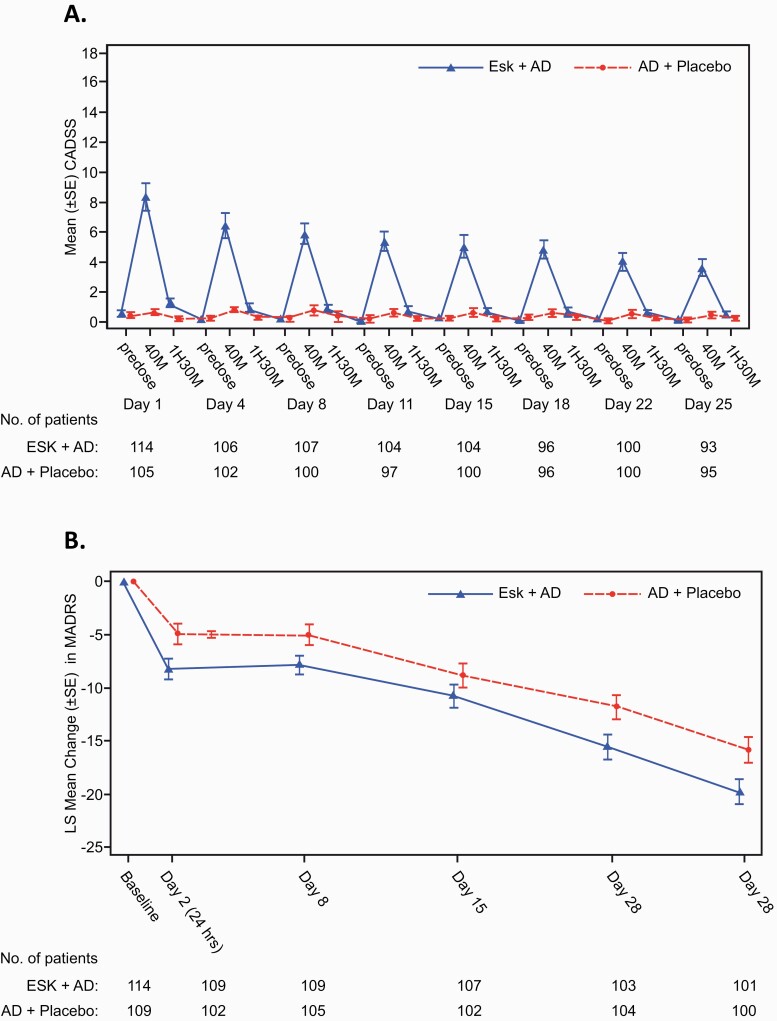
In TRANSFORM-2, the peak mean CADSS total score in patients treated with ESK decreased over time with consecutive doses; the main effect of study day and the interaction of day × treatment type were significant (P < .0001; Figure 2A). The results for changes in MADRS total scores (LS mean [95% CI] treatment difference) from baseline favored ESK + AD over AD + placebo at day 2 (−3.3 [−5.75, −0.85]), day 8 (−2.9 [−5.17, −0.59]), day 15 (−2.0 [−4.78, 0.82]), day 22 (−3.8 [−6.87, −0.65]), and day 28 (−4.0 [−7.31, −0.64]); the 95% CI did not contain 0 at any time point except for day 15. For both treatment groups, over one-third of the improvement from baseline occurred at day 2 (24 hours), and both groups continued to improve to the end of the DB induction phase (day 28) (Figure 2B). However, the mean difference between groups did not significantly differ across assessments between day 2 and 28 (P = .5341 for the interaction of day × treatment type) (Figure 2B). Observations from TRANSFORM-1 were similar, favoring the ESK + AD group (supplementary Figure 2).
Figure 2.
Temporal profiles of dissociation symptoms and antidepressant effects in the TRANSFORM-2 study cohort. (A) Dissociation symptoms assessed using the Clinician-Administered Dissociative States Scale (CADSS) total score over repeated dosing. Data are mean ± SE. Significant effects of day (P < .0001), treatment type (P < .0001), and day × treatment type (P < .0001) were detected. (B) Mean changes in depression severity from baseline assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) over repeated dosing. Significant effects of day (P < .0001) and treatment type (P = .0049), but not day × treatment type (P = .5341) were detected. Data are least squares (LS) mean ± SE. MADRS. AD, antidepressant; ESK, esketamine nasal spray; H, hour; M, minute; SE, standard error.
DISCUSSION
The data presented herein do not support a correlation between dissociative symptoms and antidepressant effects when using ESK (56 or 84 mg) in conjunction with a newly initiated oral AD in patients with TRD. The 4 lines of evidence for lack of association are as follows: (1) many patients with robust antidepressant response did not experience dissociation (CADSS total score >4) and the proportion of treatment responders did not differ significantly between patients who did vs those who did not manifest dissociation (based on total CADSS scores above vs below 4, respectively) at day 1 (first ESK dose) or day 25 (last dose); (2) there was no significant correlation between the peak increases in dissociative symptom severity (either as rated using the CADSS total score or the CADSS component scores at 40 minutes post-dose) and the improvement in depressive symptoms rated using the MADRS score at either day 2 or day 28; (3) there was no significant mediating effect of dissociation on the antidepressant effects of ESK; and (4) dissociative symptoms attenuated over repeated dosing while the improvement in depressive symptoms measured by the differential change in MADRS score between the ESK + AD group and the AD + placebo group persisted over time without evidence of decreasing.
Of the previous studies that evaluated the relationship between antidepressant effects and dissociative symptoms following administration of ketamine (0.5 mg/kg i.v.) in patients with TRD, 2 (from the same research group) reported a significant correlation between the ketamine-induced dissociative symptoms and the antidepressant effect size (Luckenbaugh et al., 2014; Niciu et al., 2018), whereas 2 others found no association between these ratings (Valentine et al., 2011; Murrough et al., 2013). Following a single ketamine infusion, Luckenbaugh et al. observed correlations between improvements in Hamilton Depression Rating Scale scores (at 230 minutes and 7 days post-dose) and dissociative symptom ratings at 40 minutes on CADSS total and depersonalization scores (Luckenbaugh et al., 2014). A second study from the same research group [patient sample overlapping with the Luckenbaugh et al. study (Luckenbaugh et al., 2014)] also reported that the changes in CADSS scores obtained at 40 minutes after a single dose of ketamine infusion significantly correlated with improvements in Hamilton Depression Rating Scale scores assessed at both 230 minutes post-dose (P = .007) and at day 7 (P = .01) in 126 patients with treatment-resistant MDD or bipolar disorder (Niciu et al., 2018). Contradicting these findings, however, 2 earlier reports [n = 24 (Murrough et al., 2013); n = 10 (Valentine et al., 2011)] detected no significant association between ketamine-induced dissociative symptoms and antidepressant effects.
The current findings in 3 large cohorts of patients with TRD treated with ESK + AD in a short-term (TRANSFORM-2) (Popova et al., 2019) and a long-term maintenance (SUSTAIN-1) (Daly et al., 2019) study did not show a significant association between dissociation and antidepressant response; these findings were further corroborated by observations from another short-term study in patients with TRD (TRANSFORM-1) (Fedgchin et al., 2019). The lack of association between ESK-induced dissociation and antidepressant response from the correlation analyses was supported by outcomes from the responder distribution, mediation, and temporal profile analyses. These convergent data showed no association between dissociative and antidepressant effects that manifested while using ESK in patients with TRD, which is in contrast to some, but not other, reports that conducted correlation analyses of similar data obtained following the ketamine i.v. treatment (Luckenbaugh et al., 2014; Niciu et al., 2018) discussed above.
Notably, the dissociation induced by the first ESK administration appeared lower in magnitude (based on CADSS scores) than that reported following the single i.v. infusion of ketamine, with the highest CADSS scores on average ranging from 6 to 8 for initial ESK administrations (Figure 2; supplementary Figure 2) compared with approximately 25 after initial administration of ketamine 0.5 mg/kg i.v. infused over 40 minutes (Luckenbaugh et al., 2014). Furthermore, only a small number of patients had a CADSS score >25 in the TRANSFORM-2 and TRANSFORM-1 cohorts (Figure 1; supplementary Figure 1). These findings appear consistent with findings from a study comparing putatively equipotent (for NMDAR antagonism) doses of esketamine (0.25 mg/kg i.v.) and ketamine (0.5 mg/kg i.v.), in which patients with TRD in the esketamine group had numerically lower median CADSS scores compared with those in the ketamine group (Mello et al., 2021). Differences in the pharmacokinetic profiles obtained using differing formulation types or routes of administration (i.v. vs nasal spray) may contribute to the differential CADSS scores observed. Moreover, differences in the magnitude and variance of the CADSS conceivably may influence the statistical sensitivity for detecting a relationship between antidepressant and dissociative effects in studies using esketamine nasal spray vs ketamine i.v.
Cumulatively, the findings regarding the association between dissociation and antidepressant effects should be interpreted within the context of several differences that are noted between the studies of ketamine i.v. and esketamine nasal spray, including the stereochemistry of the compounds, route of administration, dosing frequency, presence of adjunctive antidepressant treatments, and rating scales used to assess change in depression severity. Furthermore, differences existed in the timing of the clinical assessments between the present analysis and those reported in the Luckenbaugh and Niciu studies (Luckenbaugh et al., 2014; Niciu et al., 2018). Of note, in the single administration ketamine i.v. study (Luckenbaugh et al., 2014), dissociation observed immediately after the ketamine administration was found to be significantly associated with antidepressant response at day 7 after the infusion. In the TRANSFORM-1 and TRANSFORM-2 studies, patients received ESK dosing twice a week for 4 weeks, with MADRS ratings conducted at day 2 and thereafter on a weekly basis post baseline. Given this design difference, it was not possible to assess the association between dissociation response immediately after the first ESK dosing only and the antidepressant response 6-7 days later in the absence of intervening esketamine doses. In our analysis, we instead sought to evaluate the association between post-dose changes in CADSS total and component scores immediately after first and last dosing and MADRS total scores at day 2 (the day after the first nasal spray treatment) and day 28 (the day of last MADRS assessment of the induction phase). We selected these timepoints based on the distinct temporal courses of dissociative and antidepressant responses over repeated dosing. As shown in Figure 2, the magnitude of the CADSS ratings was highest at Tmax for ESK (approximately 40 minutes), but the peak CADSS score declined across sessions. CADSS scores after first dosing thus generally captured the initial and on average most robust dissociation responses, whereas CADSS score after last dosing reflected an attenuation of the dissociation responses. In contrast, the MADRS score reductions at day 2 (24 hours after the initial ESK administration) and day 28 measured the initial and on average greatest antidepressant responses, respectively, although the mean difference between the ESK and active control groups did not significantly change between the day 2 and day 28 assessments.
Neither the current post-hoc analysis nor the primary phase 3 clinical trials were designed to address mechanistic questions, and therefore the present findings have limited interpretation regarding target engagement. However, the extant evidence suggests both the antidepressant and dissociative effects of ESK are mediated via NMDAR antagonism (Saad et al., 2020), as supported by direct (Stone et al., 2008) and indirect (Esterlis et al., 2018) brain imaging measures reflecting NMDAR engagement. Also consistent with the conclusion that NMDAR antagonism mediates the antidepressant effects of ketamine and esketamine, the antidepressant dose ratio of esketamine vs ketamine approximates their equipotent ratio for NMDAR antagonism based on Ki values (approximately 1:2) (Mello et al., 2021). For example, the magnitude of the antidepressant effect of 0.2 mg/kg esketamine i.v. (Singh et al., 2016a) is comparable with that of 0.5 mg/kg ketamine i.v. (Singh et al., 2016b), and a randomized trial that directly compared the antidepressant efficacy of 0.25 mg/kg esketamine i.v. vs 0.5 mg/kg ketamine i.v. showed non-inferiority as well as comparable antidepressant and adverse effects between study arms (Correia-Melo et al., 2020). Finally, the involvement of NMDARs in producing antidepressant and dissociative effects was corroborated by evidence that traxoprodil (CP-101606), a NR2B selective NMDAR antagonist, produced antidepressant and (dose-dependent; see below) dissociative effects in patients with TRD that appeared similar to those reported in studies using ketamine or esketamine (Preskorn et al., 2008). Therefore, the extant data support the hypothesis that NMDAR antagonism mediates the antidepressant effects of esketamine.
Nevertheless, a recent preclinical study hypothesized that the hyperpolarization-activated cyclic-nucleotide-gated potassium channel 1 (HCN1) mediates the dissociative effects of ketamine (Vesuna et al., 2020). The reported affinity of ketamine and esketamine for HCN1 is several folds lower than their affinities for NMDAR (Vesuna et al., 2020), and it remains unclear whether ketamine and esketamine directly interact with HCN1 at their antidepressant doses in humans. The mechanism of dissociation proposed for ketamine and esketamine is different from the mechanism of action proposed for psychedelics such as psilocybin and 3,4-ethylenedioxymethamphetamine, which are under evaluation for possible efficacy in psychiatric disorders, including depression (Reiff et al., 2020). For esketamine, the results from the current study showed that the dissociative effects are neither necessary for achieving nor correlated with the antidepressant effects.
A crucial clinical implication of our data is that dose selection and titration during esketamine treatment should focus on the antidepressant response and the balance between efficacy and tolerability. In contrast, dose selection should not focus on producing dissociative symptoms to ensure that an adequate therapeutic has been administered. As illustrated in Figure 1 and supplementary Figure 1, some patients developed dissociation without adequate antidepressant response, whereas others achieved robust antidepressant responses without evidence of dissociation. Moreover, the association between dissociative and antidepressant effects changed during repeated dosing, as the severity and frequency of dissociation diminished while the antidepressant effect persisted across the 4-week DB period. In addition, studies assessing the relationships between antidepressant and dissociative effects at different esketamine doses (0.2 and 0.4 mg/kg i.v.) showed comparable antidepressant effects at both doses while the severity of dissociation increased at the higher dose (Singh et al., 2016a). Similarly, traxoprodil also showed at least partial separation between the antidepressant and dissociative effects at distinct dose levels (Preskorn et al., 2008). Of the 2 doses tested (0.75 mg/kg per hour for 1.5 hours followed by 0.15 mg/kg per hour for 6.4 hours vs 0.5 mg/kg per hour for 1.5 hour), the higher dose produced prominent dissociative effects, prompting the switch to the lower dose, which produced antidepressant effects but minimal dissociative effect (Preskorn et al., 2008). Taken together, these data imply that the presence of dissociative effects is not needed to ensure the adequacy of antidepressant dosing in individual patients receiving ESK for TRD.
The transient increases in CADSS scores following the administration of AD + placebo in the control arm of TRANSFORM-2 merit contextualization, with literature from other areas of medicine indicating that the adverse events listed in informed consent documents influenced the types of adverse events reported by study participants randomized to the placebo arm of DB clinical trials. In the AD + placebo group of TRANSFORM-2, approximately 4% of patients were rated as having CADSS scores >4. Transient dissociative side effects also were reported by some participants who received placebo in a ketamine i.v. study in patients with TRD (Acevedo-Diaz et al., 2020). The dissociative effects reported in some participants who received AD + placebo in esketamine studies may reflect the “nocebo” effect (i.e., in which a patient who receives placebo develops side effects or symptoms that can occur with the active study drug just because the patient expects them to occur). The nocebo phenomenon had been well characterized in studies performed across many areas of medicine (Myers et al., 1987; Chavarria et al., 2017; Dodd et al., 2019).
Some limitations of the current post-hoc analysis merit comment. The CADSS was developed for psychiatric disorders such as posttraumatic stress disorder and may not be sensitive to detect change in dissociative symptoms induced by ketamine or other NMDAR antagonists (Bremner et al., 1998). Currently, the optimal CADSS score threshold has not been established for detecting the presence or absence of dissociation in a population of patients with TRD receiving ESK (Williamson et al., 2019). Therefore, to investigate the impact of using different CADSS thresholds, additional analyses were performed using CADSS cutoffs of ≤2 and ≤8 (supplementary Figure 3). Both cutoffs showed similar results as with the ≤4 threshold, supporting the absence of an association between the antidepressant response and dissociation, irrespective of the threshold applied for identifying dissociation. Furthermore, other analyses (e.g., correlation, mediation, and temporal analyses) that were independent of the CADSS cutoff also demonstrated no significant association between antidepressant and dissociative effects. Notably, these negative results also extended to the subcomponent score of the CADSS (depersonalization; Table 1) reported to be predictive of antidepressant response in a previous study of ketamine (Zarate et al., 2006).
Another significant limitation of the methods is the noise introduced by both the placebo effect (which impacts both the active treatment and control arms) and the small treatment effect (which is driven by biological heterogeneity inherent within the clinical population as well as the limited sensitivity and specificity of the clinical outcome measure) (Preskorn 2008, 2014). Thus, the sensitivity for detecting inter-relationships between 2 pharmacodynamic effects assessed using clinical rating scales in antidepressant trials is limited (Preskorn 2008, 2014). In addition, the relatively small sample sizes of the ESK + AD group in the individual studies limited sensitivity for detecting weak correlations. To mitigate this latter limitation, we performed a correlational analysis of the combined data from TRANSFORM-1 and TRANSFORM-2 (supplementary Table 6). These results corroborated the findings from the individual studies in supporting the absence of correlation.
In summary, post-hoc analyses from the TRANSFORM-1 and TRANSFORM-2 short-term studies of ESK + AD (Fedgchin et al., 2019; Popova et al., 2019) in patients with TRD did not support a correlation between the severity or presence of dissociative symptoms and the antidepressant treatment response to ESK. In addition, there was no evidence of a mediation effect of dissociation on time to depression relapse based on the analysis of data from the SUSTAIN-1 relapse prevention maintenance study (Daly et al., 2019). Additional research is needed to elucidate the presumably distinct modes of action through which the NMDAR antagonist mechanism mediates the antidepressant response relative to the dissociative effects of esketamine.
Supplementary Material
Acknowledgments
Medical writing support was provided by Priya Ganpathy, MPharm, CMPP (SIRO Clinpharm Pvt. Ltd, India); this assistance was funded by Janssen Research and Development. Additional editorial assistance was provided by Harry Ma, PhD, CMPP (Janssen Global Services, LLC). The authors thank the study participants and the investigators for their participation in this study.
The study was supported by funding from Janssen Research & Development, LLC. The sponsor also provided funding for development of this manuscript.
Interest Statement
All authors are employees of Janssen Research & Development and may hold stock or stock options of Johnson and Johnson. J.B.S. and M.L.F. were employees of Janssen Research & Development, LLC at the time of study.
References
- Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CA, Park LT (2020) Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord 263:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Zarate CA Jr (2020) The role of dissociation in ketamine’s antidepressant effects. Nat Commun 11:6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, Brutsche NE, Ameli R, Furey ML, ZarateCA, Jr (2014) Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res 58:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Luckenbaugh DA, Richards EM, Walls TL, Brutsche NE, Ameli R, Niciu MJ, Vande Voort JL, ZarateCA, Jr (2015) Assessing measures of suicidal ideation in clinical trials with a rapid-acting antidepressant. J Psychiatr Res 68:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM (1998) Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11:125–136. [DOI] [PubMed] [Google Scholar]
- Chavarria V, Vian J, Pereira C, Data-Franco J, Fernandes BS, Berk M, Dodd S (2017) The placebo and nocebo phenomena: their clinical management and impact on treatment outcomes. Clin Ther 39:477–486. [DOI] [PubMed] [Google Scholar]
- Correia-Melo FS, et al. (2020) Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: a randomized, double-blind, non-inferiority study. J Affect Disord 264:527–534. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, et al. (2019) Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 76:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, ZarateCA, Jr (2010a) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, ZarateCA, Jr (2010b) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd S, Walker AJ, Brnabic AJM, Hong N, Burns A, Berk M (2019) Incidence and characteristics of the nocebo response from meta-analyses of the placebo arms of clinical trials of olanzapine for bipolar disorder. Bipolar Disord 21:142–150. [DOI] [PubMed] [Google Scholar]
- Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, Yang J, Pittenger C, Sanacora G, Krystal JH, Parsey RV, Carson RE, DeLorenzo C (2018) Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C]ABP688 and PET imaging study in depression. Mol Psychiatry 23:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, Vitagliano D, Blier P, Fava M, Liebowitz M, Ravindran A, Gaillard R, Ameele HVD, Preskorn S, Manji H, Hough D, Drevets WC, Singh JB (2019) Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol 22:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, Hough D, Manji H, Drevets WC, Canuso CM (2020) Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry 81:19m13191. [DOI] [PubMed] [Google Scholar]
- Hu YD, Xiang YT, Fang JX, Zu S, Sha S, Shi H, Ungvari GS, Correll CU, Chiu HF, Xue Y, Tian TF, Wu AS, Ma X, Wang G (2016) Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med 46:623–635. [DOI] [PubMed] [Google Scholar]
- Imai K, Keele L, Tingley D (2010) A general approach to causal mediation analysis. Psychol Methods 15:309–334. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Bentley KH, Eikermann M, Taylor N, Akeju O, Swee MB, Pavone KJ, Petrie SR, Dording C, Mischoulon D, Alpert JE, Brown EN, Baer L, Nock MK, Fava M, Cusin C (2019) Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: a randomized, double blind, placebo controlled trial. J Affect Disord 243:516–524. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, Hough D, Drevets WC, Manji H, Canuso CM (2021) Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol 24:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson & Johnson, Press Release (2020) Janssen announces U.S. FDA approval of SPRAVATOR (esketamine) CIII nasal spray to treat depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. [Google Scholar]
- Lange T, Hansen JV (2011) Direct and indirect effects in a survival context. Epidemiology 22:575–581. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA (2014) Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord 159:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello RP, Echegaray MVF, Jesus-Nunes AP, Leal GC, Magnavita GM, Vieira F, Caliman-Fontes AT, Telles M, Guerreiro-Costa LNF, Souza-Marques B, Bandeira ID, Santos-Lima C, Marback RF, Correia-Melo FS, Lacerda ALT, Quarantini LC (2021) Trait dissociation as a predictor of induced dissociation by ketamine or esketamine in treatment-resistant depression: secondary analysis from a randomized controlled trial. J Psychiatr Res 138:576–583. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Cairns JA, Singer J (1987) The consent form as a possible cause of side effects. Clin Pharmacol Ther 42:250–253. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Shovestul BJ, Jaso BA, Farmer C, Luckenbaugh DA, Brutsche NE, Park LT, Ballard ED, Zarate CA Jr (2018) Features of dissociation differentially predict antidepressant response to ketamine in treatment-resistant depression. J Affect Disord 232:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, Hough D, Manji H, Drevets WC, Sanacora G, Steffens DC, Adler C, McShane R, Gaillard R, Wilkinson ST, Singh JB (2020) Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry 28:121–141. [DOI] [PubMed] [Google Scholar]
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB (2019) Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry 176:428–438. [DOI] [PubMed] [Google Scholar]
- Preskorn SH (2008) Flat dose-response curves for efficacy: what do they mean to the clinician? J Psychiatr Pract 14:232–236. [DOI] [PubMed] [Google Scholar]
- Preskorn SH (2014) Therapeutic Drug Monitoring (TDM) in psychiatry (part I): why studies attempting to correlate drug concentration and antidepressant response don’t work. J Psychiatr Pract 20:133–137. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, Kalin NH, McDonald WM; the Work Group on Biomarkers and Novel Treatments, a Division of the American Psychiatric Association Council of Research (2020) Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry 177:391–410. [DOI] [PubMed] [Google Scholar]
- Saad Z, Hibar D, Fedgchin M, Popova V, Furey ML, Singh JB, Kolb H, Drevets WC, Chen G (2020) Effects of Mu-Opiate receptor gene polymorphism rs1799971 (A118G) on the antidepressant and dissociation responses in esketamine nasal spray clinical trials. Int J Neuropsychopharmacol 23:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L (2016a) Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry 80:424–431. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L (2016b) A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 173:816–826. [DOI] [PubMed] [Google Scholar]
- Spravato (esketamine) nasal spray. Prescribing information. 2020. Janssen Pharmaceutical Companies. [Google Scholar]
- Stone JM, Erlandsson K, Arstad E, Squassante L, Teneggi V, Bressan RA, Krystal JH, Ell PJ, Pilowsky LS (2008) Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [(123)I]CNS-1261 SPET study. Psychopharmacology 197:401–408. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G (2011) The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res 191:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesuna S, Kauvar IV, Richman E, Gore F, Oskotsky T, Sava-Segal C, Luo L, Malenka RC, Henderson JM, Nuyujukian P, Parvizi J, Deisseroth K (2020) Deep posteromedial cortical rhythm in dissociation. Nature 586:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajs E, et al. (2020) Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry 81:19m12891. [DOI] [PubMed] [Google Scholar]
- Williamson D, Turkoz I, Wajs E, Aluisio L, Singh JB, Starr HL, Daly EJ (2019) Linking adverse events of dissociation reported with esketamine dosing by clinician-perceived severity and CADSS descriptors. In: The International Society for CNS Clinical Trials and Methodology (ISCTM) 15th Annual Scientific Meeting, February 19–21, 2019, Washington DC. [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.