Figure 1. Exogenous mitochondria restore aspartate levels in CD4+ T cells to limit production of TNF-α and joint inflammation in rheumatoid arthritis-like disease.
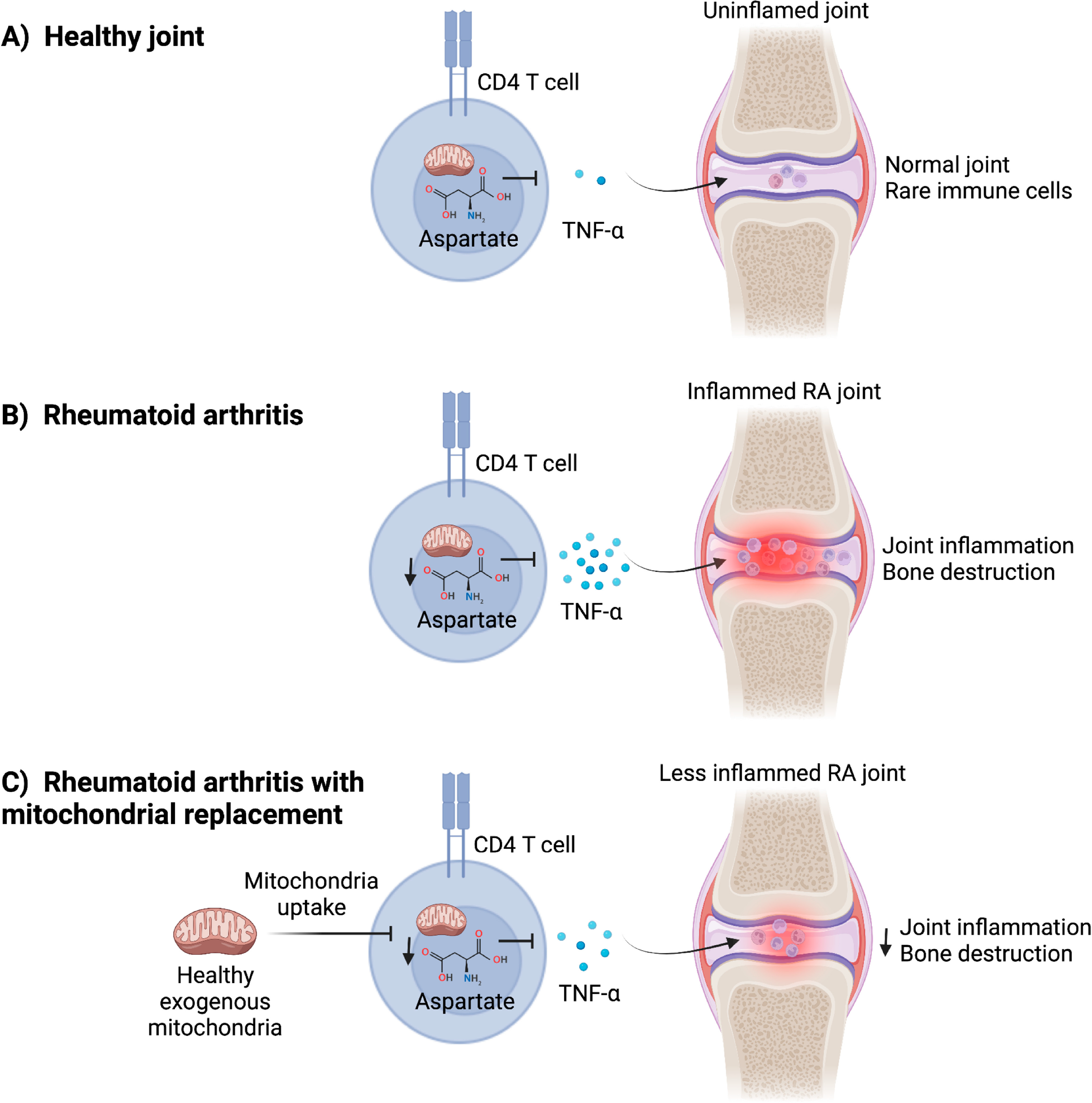
(A) In the healthy joint, CD4+ T cells are rare and have normal mitochondrial metabolism that allows adequate concentrations of citric acid cycle intermediary metabolites, such as aspartate, to be produced. Aspartate suppresses production of proinflammatory cytokines, such as Tumor necrosis factor (TNF)-α. (B) CD4+ T cells from rheumatoid arthritis (RA) patients have reduced mitochondrial function and decreased concentrations of aspartate. The inhibitory effect of aspartate on TNF-α production is removed, leading to upregulated expression of this cytokine to drive inflammation and bone destruction in RA-afflicted joints. (C) Treating RA patient-derived T cells with healthy exogenous mitochondria restores mitochondrial metabolism in T cells, increases aspartate concentrations, and inhibits expression of TNF-α and other pro-inflammatory cytokines. Ex vivo mitochondria treatment of RA T cells attenuated joint inflammation and bone destruction in a humanized mouse model of RA-like disease, suggesting that mitochondria transfer could be utilized to “metabolically engineer” T cells for the treatment of rheumatologic diseases, such as RA.
