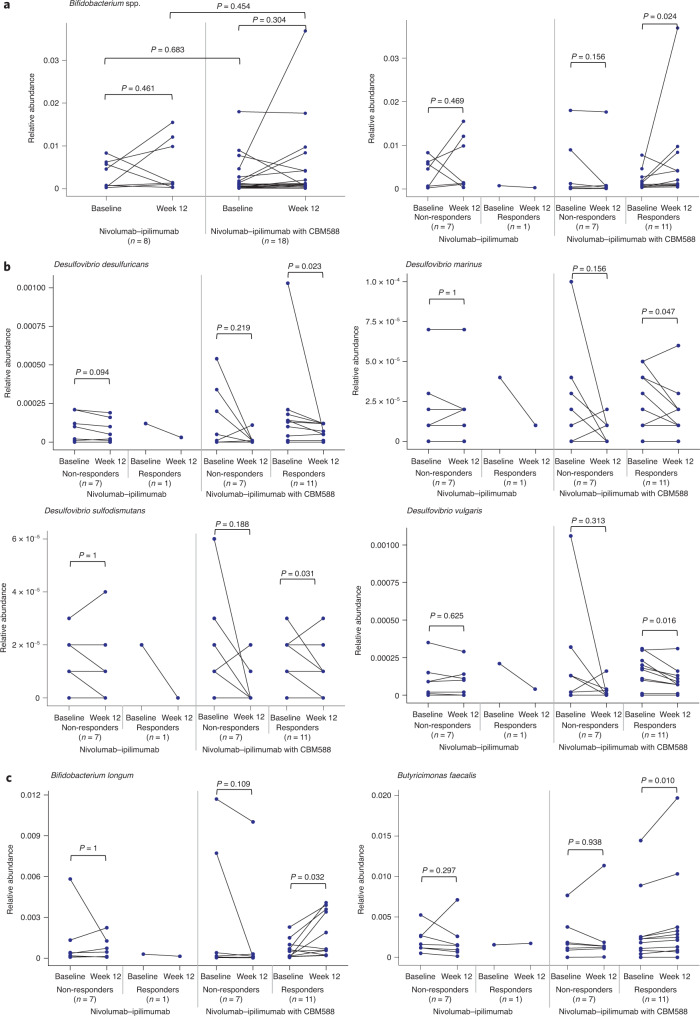
Fig. 2. Microbiome assessment in patients with mRCC treated with nivolumab–ipilimumab with or without CBM588.
a, Change in Bifidobacterium spp. from baseline to week 12 in patients by treatment arm, and by treatment arm and response. b,c, Decreases (b) and increases (c) in relative abundance of gut microbiome species associated with response to nivolumab–ipilimumab with CBM588. Analyses were performed using n = 52 stool samples from n = 26 patients (n = 18 patients in the nivolumab–ipilimumab with CBM588 arm and n = 8 patients in the nivolumab–ipilimumab arm). The Wilcoxon signed rank test was used to perform comparisons between two timepoints within the same treatment arm and the Mann–Whitney U test was used for comparisons between the two arms.