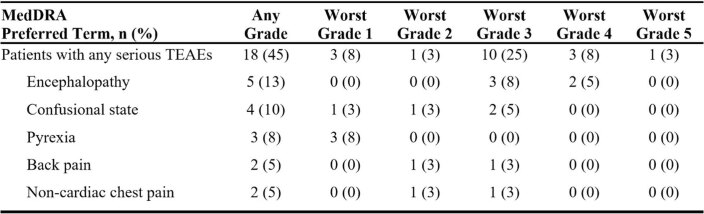
Extended Data Table 4.
Serious adverse events occurring in at least two treated patients (n = 40)
TEAE includes all AEs with onset on or after axicabtagene ciloleucel infusion date. AEs with onset during retreatment period are excluded. Multiple incidences of the same AE in one patient are counted once at the worst grade for that patient. Preferred terms are sorted in descending order of frequency count in any grade. AEs are coded using MedDRA v.23.1 and graded according to CTCAE v.5.0. AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Event; MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse event.