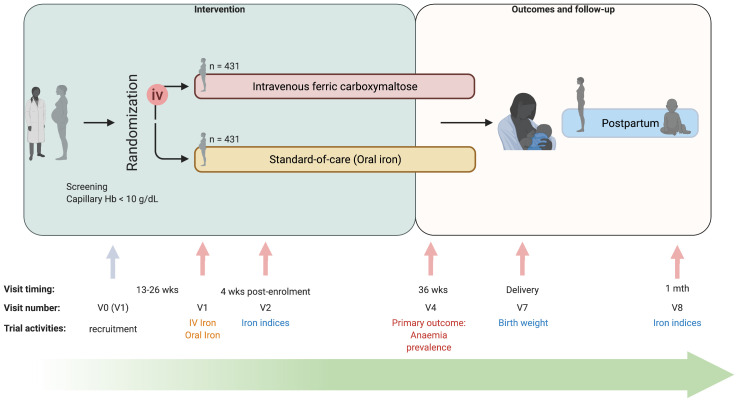
Figure 1. Study design and timeline of the Randomized controlled trial of the Effect of intraVenous iron on Anaemia in Malawian Pregnant women (REVAMP) (created with BioRender).
V0 – visit 0; V1 – Visit 1; V2 – Visit 2; V4 – Visit 4; V7 – Visit 7; V8 – Visit 8; Abbreviations: iv – intravenous; wks – weeks. The study was designed as a two-arm trial (intravenous ferric carboxymaltose versus standard-of-care (oral iron)) where women were randomized in their second trimester of pregnancy. Study visits occurred over pregnancy, at birth, and follow-up to one-month postpartum. Women were scheduled to be visited in their home at 34 weeks’ gestation (Visit 3), and every two weeks from 38 weeks’ gestation until delivery (Visit 5 and 6) to measure capillary Hb, not included in the study design schema.