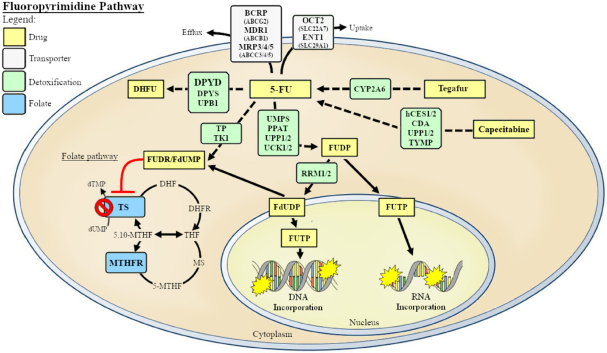
Figure 1.
Metabolic pathway of fluoropyrimidines. 5-Fluorouracil (5-FU), is metabolized intracellularly to its active form 5-fluoro-2-deoxyuridine-5’-monophospate (5-FdUMP) through two consecutive reactions catalyzed by thymidine phosphorylase (TP) and thymidine kinase (TK). Another important metabolic enzyme is the ribonucleotide reductase, composed of large subunit RRM-1 and small subunit RRM-2, that converts fluorouridine diphosphate (FUDP) to fluorodeoxyuridine diphosphate (FdUDP), which preferentially affects DNA metabolism. 5-FU carries on his cytotoxic effect by mediating the formation of an inhibitory ternary complex, involving its metabolite 5-FdUMP, thymidylate synthase (TS, TYMS) and 5,10-methylentetrahydrofolate (5,10-MTHF). The formation of this complex inhibits TS activity, with subsequent diminution of thymidylate levels and consequent suppression of DNA synthesis. Dihydropyrimidine dehydrogenase (DPD, DPYD) is the first and rate-limiting enzyme of the fluoropyrimidines catabolic pathway converting 5-FU to dihydrofluorouracil (DHFU) while 5,10-methylenetetrahydrofolate reductase (MTHFR) catalyzes the irreversible conversion of 5,10-MTHF, required for DNA synthesis, to 5-MTHF, the primary methyl donor indispensable for nucleic acid methylation. Human carboxylesterase isoforms 1 and 2 (hCES1/2) and cytidine deaminases (CDAs) are necessary for capecitabine activation and metabolism. UMPS encodes the enzyme orotate phosphoribosyltransferase (OPRT), which catalyzes the conversion of 5-FU into fluorouridine monophosphate (FUMP), a common substrate for the production of cytotoxic metabolites that target RNA and DNA. Some ATP-binding cassette (ABC) and solute carrier (SLC) membrane transporter are involved in drug translocation of the drug