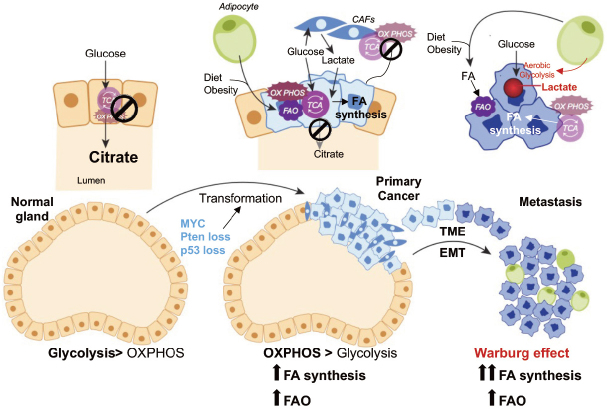
Figure 1.
Metabolic alterations during PCa progression. Diagramatic representation of putative metabolic changes during progression from non-neoplastic disease to mCRPC. In normal prostate epithelial cells, the tricarboxylic acid cycle (TCA) cycle is truncated, and low rate of oxidative phosphorylation (OXPHOS) are observed. High levels of citrate are released in the seminal liquid. During malignant transformation, which is induced by genetic alterations (i.e., Pten loss, p53 loss, MYC overexpression), PCa cells reactivate the TCA cycle to oxidize citrate for energy production and convert citrate to acetyl-CoA for de-novo lipid synthesis. Fatty acids (FAs) from diet or obesity-associated adipocyte lipolysis also feed into the TCA cycle for energy production. Metabolic crosstalk is observed between cancer-associated fibroblasts (CAFs) and PCa cells, whereby CAFs provide lactate as fuel source. Increased aerobic glycolysis or the Warburg effect is observed in the metastatic stages of the disease. The Warburg effect seems to be stimulated by adipocytes in the bone marrow (BM). BM adipocytes promote the expression of glycolytic enzymes in mCRPC cells. Increased lactate secretion in TME is associated with tumor aggressiveness and metastases formation. Both de-novo lipogenesis and FAO are observed in the metastatic niche to fulfill the energetic and anabolic needs of mCRPC cells. EMT: epithelial-mesenchymal transition; TME: tumor microenvironment; PCa: prostate cancer; mCRPC: metastatic, castration-resistant prostate cancer