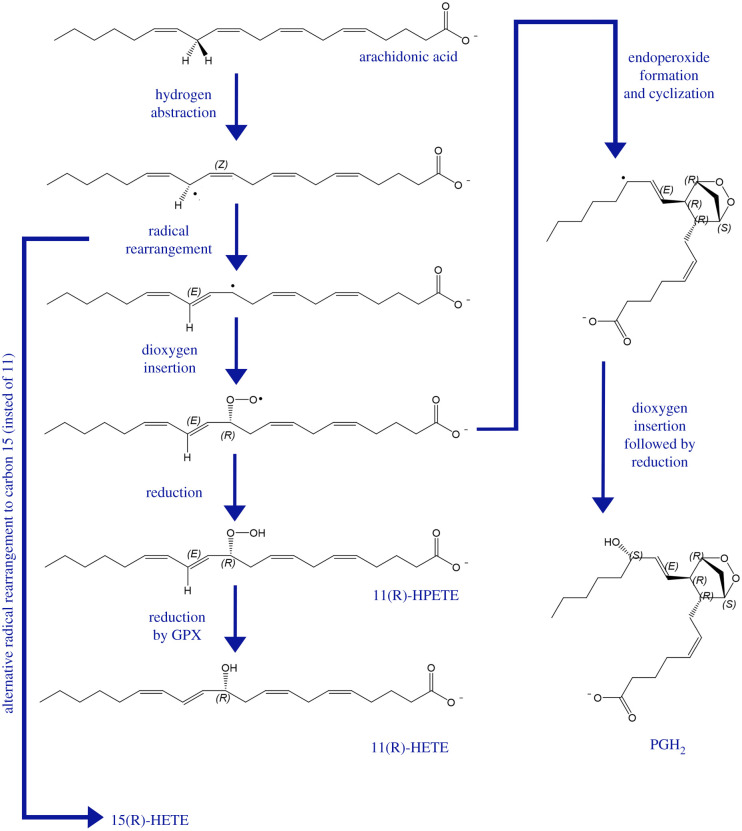
Figure 7.
Reaction mechanism of cyclooxygenase (COX) and the generation of 11-HETE and 15-HETE as by-products of the dioxygenase reactions. Arachidonic acid (AA) undergoes hydrogen atom abstraction followed by radical rearrangement to allow for the insertion of a dioxygen molecule. The resultant product undergoes endoperoxide formation and cyclization to ultimately generate prostaglandin H2 (PGH2)—the intermediary product of COX-derived prostaglandins and thromboxanes. The dioxygenated AA may also undergo reduction instead of cyclization, leading to the generation of 11(R)-HPETE and subsequent reduction by glutathione peroxidase (GPX) enzymes to 11(R)-HETE. Alternative radical rearrangement to carbon 15 (instead of 11) can lead to the generation of 15(R)-HETE downstream of COX metabolism of AA. HPETE, hydroperoxyeicosatetraenoic acid; HETE, hydroxyeicosatetraenoic acid. Adapted from Hajeyah et al. [58].