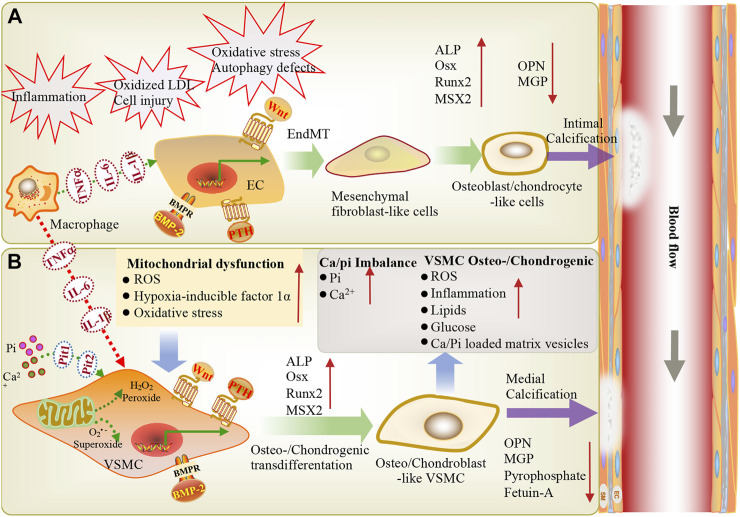
FIGURE 1.
During inflammation, macrophages infiltrate through EC adhesion and migration across endothelial cells, resulting in macrophage activation and the release of inflammatory cytokines (TNF-α, IL-6, IL-1β), further stimulating VSMCs and ECs, eventually leading to vascular calcification. Vascular endothelial cells exposed to shear stress induced by blood flow are more sensitive to shear stress-induced EndMT and BMP-induced osteogenic differentiation, thereby triggering endothelial damage and inducing vascular calcification. (A) Endothelial cells can be stimulated via inflammation, oxidative LDL aggregation, cell injury, oxidative stress, and autophagy defects. BMP, PTH and Wnt signaling are involved in endothelial mesenchymal transformation, the differentiation of endothelial cells into mesenchymal fibroblasts, and their further differentiation into osteoblast-like cells. These changes are accompanied by a high expression of osteogenic genes (ALP, Runx2, BMP2, MSX2) and low expression of the calcification inhibitor MGP, leading to intimal calcification. (B) Ca2+/Pi imbalance and mitochondrial dysfunction lead to reactive oxygen species production and oxidative stress. BMP, Wnt, and PTH signaling induce the osteogenic/chondrogenic differentiation of vascular smooth muscle cells, ultimately leading to medial calcification. EC: endothelial cells; VSMC: vascular smooth muscle cell; ALP: alkaline phosphatase; Pit1, Pit2: NaPi cotransporters; LDL: low-density lipoprotein; OPN: osteopontin; PTH: parathyroid hormone; Runx2: runt-related transcription factor-2; Msx2: msh homeobox-2; IL1-β: interleukin-1 beta; IL-6: interleukin-6; Osx: osterix; EndMT: endothelial-mesenchymal transition; BMP: Bone morphogenetic protein; ROS: Reactive oxygen species; TNF-α: tumor necrosis alpha; Pi: inorganic phosphate.