Abstract
Intranodal palisaded myofibroblastoma (IPM) is a rare, benign mesenchymal neoplasm of the lymph nodes with a broad differential diagnosis. We report a case of an 82-year-old woman presenting with a slow growing, right inguinal mass. The tumor arose as a circumscribed neoplasm inside a lymph node and consisted of bland spindle cells with nuclear palisading and intervening areas of amianthoid-like fibers among interstitial hemorrhage and hemosiderin-laden histiocytes in the stroma, typical histomorphological characteristics of IPM. Immunohistochemically, the neoplastic cells were positive for vimentin, smooth muscle actin (SMA), β-catenin, cyclin D1 and discovered on gastrointestinal stromal tumor (GIST) 1 (DOG1) immunostainings. A literature review and differential diagnosis of IPM are discussed. To the best of our knowledge, this is the first case of DOG1 immunoexpression in a case of IPM.
Keywords: mesenchymal neoplasm, spindle cell tumor, amianthoid fiber, lymph node
⧉ Introduction
First described in 1989 by different teams of researchers [1,2,3], intranodal palisaded myofibroblastoma (IPM), also denominated as intranodal hemorrhagic spindle cell tumor with amianthoid fibers and as solitary spindle cell tumor with myoid differentiation of the lymph node, is a rare benign mesenchymal neoplasm of lymph nodes. IPM is characterized by spindle cell population with palisaded nuclei and intervening stellate, round or elongated hyalinizing areas of (amianthoid-like) collagen fibers. This characteristic morphology along with the immunohistochemical (IHC) profile set the diagnosis.
From the first description of IPM, fewer than 100 cases of IPM have been reported in the literature [4]. The overwhelming majority of the cases arises in inguinal lymph nodes, with limited cases emerging in lymph nodes of submandibular [5], cervical [6], axillary [7], mediastinal [8], para-esophageal [9] and retroperitoneum [10] region, and presents as a gradually developing, painless, infrequently tender, mass or discovered incidentally during work-up for another cause [10]. There are at least two cases where the lesion was multicentric, affecting more than one inguinal lymph node [4, 11]. The age of the patients ranges from 18 months [11] to 84 years old [12], showing a male predominance and no ethnic predilection [13].
Aim
Herein, we present a case of IPM with DOG1 immunoexpression, the first reported to our knowledge, along with an incidental finding of a Pacinian-like structure with IHC features of a Renaut body in an adjacent peripheral nerve.
⧉ Case presentation
The patient, an 82-year-old woman, presented in the surgical outpatient clinic complaining of right painless inguinal swelling. She reported no history of trauma or prior surgery in the region. On physical examination, a firm, immobile mass was present in the right groin, without signs of inflammation. Computed tomography showed an enlarged right inguinal lymph node (Figure 1) that was decided to be surgically excised to exclude the presence of neoplasia.
Figure 1.
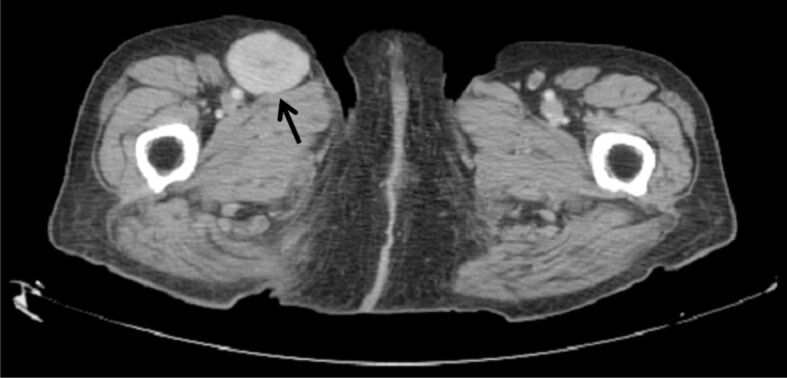
Computed tomography revealed an enlarged lymph node (arrow) in the right inguinal area
The specimen received for the histopathological (HP) examination was a firm, nodular mass, with weight of 36 g and size of 4.9×4.5×2.5 cm. Cut sections revealed a well-circumscribed mass, with a whitish/grey-white surface.
The microscopic findings showed a hypercellular spindle cell neoplasm that was completely surrounded by a collagenous capsule with variable thickness and focal hyalinization. A rim of residual lymphoid tissue was visible at the periphery of the lesion (Figure 2A). The cells had scant eosinophilic cytoplasm, with round or elongated blunt-ended nuclei, demonstrating no atypia or pleomorphism. Perinuclear vacuoles were frequently found. Mitotic activity was low (0–1 mitotic figures per 50 high-power fields). The cells were arranged in haphazardly intersecting fascicles and whorls, with often apparent nuclear palisading (Figure 2, B and C). Scattered round or stellate eosinophilic fibrotic areas, resembling amianthoid fibers (Figure 2C) and stained blue with Masson’s trichrome histochemical staining (Figure 2D), were observed between the fascicles. This staining also revealed intracellular globular salmon-colored inclusions in the neoplastic cells (Figure 2E). Areas of interstitial hemorrhage, diffusely distributed hemosiderin-laden histiocytes (Figure 2F), foci of fibrosis with hyalinization and regions of myxoid change characterized the stroma.
Figure 2.
(A) The neoplasm was surrounded by a fibrous capsule, compressing the residual lymphoid tissue of the lymph node. (B) It is composed of spindle cells with nuclear palisading (C) and intervening stellate or round eosinophilic areas, that stained blue (D) with Masson’s trichrome histochemical staining. The same staining reveals (E) intracellular salmon-colored inclusions (arrows). (F) Hemorrhagic areas and hemosiderin-laden histiocytes are found in the stroma. Hematoxylin–Eosin (HE) staining: (A and B) ×100; (C and F) ×400. Masson’s trichrome staining: (D) ×100; (E) ×400
Immunohistochemically, the cells showed positivity to vimentin, smooth muscle actin (SMA) (Figure 3A), cyclin D1 and β-catenin (strong nuclear) (Figure 3B). The IHC staining for discovered on gastrointestinal stromal tumor (GIST) 1 (DOG1) (mouse monoclonal antibody, clone IHC562, 1:100 dilution) showed diffuse membranous and cytoplasmic positivity (Figure 3C). The tumor cells demonstrated no immunoreaction to desmin, caldesmon, cytokeratin (CK) AE1/AE3, epithelial membrane antigen (EMA), cluster of differentiation (CD)34, CD31, D2-40, laminin, CD117, S100, SRY-box transcription factor 10 (SOX10) and glial fibrillary acidic protein (GFAP). The IHC staining for human herpesvirus 8 (HHV8) and the in situ hybridization for Epstein–Barr virus (EBV)-encoded small ribonucleic acid (RNA) (EBER) were also negative. The Ki67 proliferation index was low (<2%) (Figure 3D).
Figure 3.
Tumor cells show diffuse positivity to SMA (A), strong nuclear positivity to β-catenin (B) and diffuse positivity to DOG1 (C). The Ki67 proliferation index was low (<2%) (D). Anti-SMA antibody immunomarking: (A) ×100. Anti-β-catenin antibody immunomarking: (B) ×100. Anti-DOG1 antibody immunomarking: (C) ×400. Anti-Ki67 antibody immunomarking: (D) ×100. DOG1: Discovered on gastrointestinal stromal tumor (GIST) 1; SMA: Smooth muscle actin
Based on the histomorphological and IHC findings, the diagnosis of IPM was set. The patient has been followed up for a year without recurrence of the tumor.
In addition, at the periphery of a small nerve trunk adjacent to the neoplasm, an oval-shaped whorled structure attached to the perineurium was observed (Figure 4). It consisted of concentric layers of flattened cells, with elongated nuclei separated by extracellular material, resembling a Pacinian corpuscle. Immunohistochemically, the cells were positive for EMA and negative for S100 and SMA. Further evaluation with the Alcian Blue histochemical staining was impossible because the structure disappeared in deeper sections. The HP and IHC findings supported the perineurial origin of the structure and were compatible with a Renaut body.
Figure 4.

A whorled oval-shaped structure is observed in an adjacent small nerve trunk. HE staining, ×100
⧉ Discussions
IPM is a rare and relatively recently described tumor that should be aware of to avoid misdiagnosis. In 2013, Loizou et al. [13] described five key microscopic features of IPM: spindle cells with nuclear palisading, intracellular and extracellular fuchsinophilic bodies that stain positive for SMA, intraparenchymal hemorrhage and erythrocyte extravasation, the presence of amianthoid fibers and compressed remnants of lymphoid tissue at the periphery. Two years later, Laskin et al. [14], in their seminal case series of 18 patients with IPM, emphasized three prominent HP features: spindle cell fascicles with nuclear palisades, collagenous bodies composed of amianthoid-like fibers and small, rounded, salmon-colored perinuclear intracytoplasmic inclusions, which are believed to contain actin microfilaments and their presence can be accentuated by Masson’s trichrome histochemical staining and anti-actin immunostaining [14]. Our case includes all the above-mentioned features of IPM.
The spindle cells of IPM typically express immunoreactivity to vimentin, SMA, muscle specific actin, muscle myosin, and most of them to cyclin D1 and β-catenin. Occasionally, positivity to Factor XIIa [15], D2-40 [6], calponin [10] and desmin (only focal) [14] has been reported. To the best of our knowledge, our case is the first in the literature to report DOG1 immunoexpression in a case of IPM. Nevertheless, the cell of origin of IPM remains vague. Based on the IHC and electron microscopic findings, the “guilty” cell may be found between stromal cells with myoid features and modified smooth muscle cells originating from hilar blood vessels [14]. Pathogenetically, the neoplasm is driven by mutations on the catenin beta 1 (CTNNB1) gene, especially gain-of-function mutations in exon 3. Some studies have also linked the tumor with history of trauma in the region and viral infection from HHV8 or EBV [14]. In our case, the patient reported no history of trauma and the IHC testing for HHV8 and EBV (EBER) was negative.
A salient characteristic of IPM, the acellular, round or stellate areas of eosinophilic material, at first was believed to comprise amianthoid fibers. Further studies with electron microscopy revealed the width of normal collagen fibers (80–150 nm) compared to the expected width of amianthoid fibers (280–1000 nm) [16]. Immunohistochemically, type I collagen is expressed on the whole eosinophilic area, whereas type III collagen expression is restricted only at the periphery [16]. In some cases [17], small blood vessels have been noticed inside these areas, introducing the idea that condensation of perivascular collagen may result in the formation of these areas [14]. Other researchers reported grouped distribution of mast cells around these areas, hinting at a potential role of mast cells in their formation [6].
The differential diagnosis list of IPM is wide and includes mostly other spindle cell neoplasms. Schwannomas are circumscribed tumors with spindle cell morphology and nuclear palisading (Verocay bodies) in Antoni A areas and have been reported in lymph nodes, extremely rarely though [18]. Their strong S100 immunoexpression helps greatly in the differential diagnosis. Kaposi sarcoma can present with spindle cell fascicles with minimal atypia and hemorrhage and hemosiderin deposits in the stroma. Other morphological characteristics and immunoexpression of endothelial markers (CD34, CD31) avoid confusion with IPM. Spindle cell melanoma expresses S100 protein and SOX10 immunoreactivity. Leiomyosarcomas usually have pleomorphism of nuclei with apparent mitotic figures. One of the HP patterns of inflammatory myofibroblastic tumor is fascicular proliferation of SMA-positive spindle cells. The dense inflammatory background of the neoplasm though, is characteristic and different from IPM. GISTs should be considered, in cases of para-esophageal or retroperitoneum lymph nodes. The cytologically bland spindle cells, with occasional nuclear palisading and paranuclear vacuolization can be seen in both neoplasms. The possible DOG1 immunoexpression by IPM cells, as depicted in our case, may contribute to the differential diagnostic problems of this entity. However, other HP features of GISTs and the immunoreactivity to CD117 and CD34 diagnosis are immensely helpful in the discrimination of the two neoplasms. Finally, soft tissue tumors expressing β-catenin immunopositivity, comprise a potential misdiagnosis pitfall. Desmoid fibromatosis is characterized by bland fibroblasts or myofibroblasts, expressing SMA and β-catenin immunoreaction, surrounding paucicellular hyalinized areas, or densely eosinophilic collections of keloidal-type collagen, that may be misinterpreted as the amianthoid-like fibers of IPM. Nevertheless, IPM arises in lymph nodes and is surrounded by a thin capsule contrary to the localization and the infiltrative nature of desmoid fibromatosis. The intranodal nature of IPM, along with the unique characteristics of each neoplasm, is also important for the distinction between IPM and other soft tissue tumors showing beta catenin positivity, such as solitary fibrous tumor (SFT) and synovial sarcoma [19].
The second noteworthy, albeit incidental finding of this case is the presence of a Pacinian-like structure of perineurial origin in an adjacent nerve trunk, presenting HP features of Renaut body. Renaut bodies are cylindrical structures that derive from perineurium [20] and they are believed to function as pressure cushions for nerves. Its presence in our case is most likely due to the pressure of the IPM on the nearby nerve.
⧉ Conclusions
Since IPM has a benign course, with rare, reported cases of recurrence, it is of utmost importance to be differentiated from other spindle cell neoplasms, some of them malignant. Our case shows that IPM can also express DOG1 immunostaining. Pathologists should be aware of this possibility to avoid a potential diagnostic pitfall. Despite multiple studies some aspects of the pathogenesis of this rare neoplasm remain vague and require further multicenter research efforts.
Conflict of interest
Conflict of interest
The authors declare no potential conflict of interests with respect to the research, authorship, and/or publication of this article.
Informed consent
A written informed consent from the patient has been obtained.
Acknowledgments
Funding
The authors receive no financial support for the research, authorship, and/or publication of this article.
References
- 1.Weiss SW, Gnepp DR, Bratthauer GL. Palisaded myofibroblastoma. A benign mesenchymal tumor of lymph node. Am J Surg Pathol. 1989;13(5):341–346. [PubMed] [Google Scholar]
- 2.Suster S, Rosai J. Intranodal hemorrhagic spindle-cell tumor with "amianthoid" fibers. Report of six cases of a distinctive mesenchymal neoplasm of the inguinal region that simulates Kaposi’s sarcoma. Am J Surg Pathol. 1989;13(5):347–357. [PubMed] [Google Scholar]
- 3.Lee JY, Abell E, Shevechik GJ. Solitary spindle cell tumor with myoid differentiation of the lymph node. Arch Pathol Lab Med. 1989;113(5):547–550. [PubMed] [Google Scholar]
- 4.Karabulut YY, Kara T, Berkeşoğlu M. Intranodal palisaded myofibroblastoma - a rare case report and literature review. APMIS. 2016;124(10):905–910. doi: 10.1111/apm.12580. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CDM, Stirling RW. Intranodal myofibroblastoma presenting in the submandibular region: evidence of a broader clinical and histological spectrum. Histopathology. 1990;16(3):287–293. doi: 10.1111/j.1365-2559.1990.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 6.Cimpean AM, Raica M. Intranodal hemorrhagic spindle cell tumor with amianthoid fibers – report of a case with emphasis to mast cell reaction and D2-40 expression. In Vivo. 2013;27(3):395–399. [PubMed] [Google Scholar]
- 7.D’Antonio A, Addesso M, Amico P, Fragetta F. Axillary intranodal palisaded myofibroblastoma: report of a case associated with chronic mastitis. BMJ Case Rep. 2014;2014:bcr2014205877–bcr2014205877. doi: 10.1136/bcr-2014-205877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yim IHW, Will MB, Dhaliwal C, Salter DM, Walker WS. Intranodal palisaded myofibroblastoma masquerading as N2 non-small cell lung carcinoma. Ann Thorac Surg. 2016;102(1):e47–e48. doi: 10.1016/j.athoracsur.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Tipps AMP, Hasteh F, Lin G, Zare SY. Intranodal palisaded myofibroblastoma of para-esophageal lymph node: case report with cytologic and histologic findings. Diagn Cytopathol. 2019;47(12):1306–1309. doi: 10.1002/dc.24303. [DOI] [PubMed] [Google Scholar]
- 10.Sagar J, Vargiamidou A, Manikkapurath H. Intranodal palisaded myofibroblastoma originating from retroperitoneum: an unusual origin. BMC Clin Pathol. 2011;11:7–7. doi: 10.1186/1472-6890-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahimi S, Onetti Muda A, Faraggiana T. Multicentric intranodal myofibroblastoma in an infant. Histopathology. 1995;27(5):477–478. doi: 10.1111/j.1365-2559.1995.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 12.Altinbas NK, Oz I, Ustuner E, Gulpinar B, Peker E, Akkaya Z, Peker A, Ceyhan K, Yagci C. Intranodal palisaded myofibroblastoma: radiological and cytological overview. Pol J Radiol. 2016;81:342–346. doi: 10.12659/PJR.895743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loizou P, Evgeniou E, Scott-Young N, Orlando A. Intranodal palisaded myofibroblastoma presenting as lymphadenopathy of the groin. BMJ Case Rep. 2013;2013:bcr2012006374–bcr2012006374. doi: 10.1136/bcr-2012-006374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laskin WB, Lasota JP, Fetsch JF, Felisiak-Golabek A, Wang ZF, Miettinen M. Intranodal palisaded myofibroblastoma: another mesenchymal neoplasm with CTNNB1 (β-catenin gene) mutations: clinicopathologic, immunohistochemical, and molecular genetic study of 18 cases. Am J Surg Pathol. 2015;39(2):197–205. doi: 10.1097/PAS.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleist B, Poetsch M, Schmoll J. Intranodal palisaded myofibroblastoma with overexpression of cyclin D1. Arch Pathol Lab Med. 2003;127(8):1040–1043. doi: 10.5858/2003-127-1040-IPMWOO. [DOI] [PubMed] [Google Scholar]
- 16.Skálová A, Michal M, Chlumská A, Leivo I. Collagen composition and ultrastructure of the so-called amianthoid fibres in palisaded myofibroblastoma. Ultrastructural and immunohistochemical study. J Pathol. 1992;167(3):335–340. doi: 10.1002/path.1711670312. [DOI] [PubMed] [Google Scholar]
- 17.Koseoglu RD, Ozkan N, Filiz NO, Kayaoglu HA, Aydin M, Culha EN, Ersoy OF. Intranodal palisaded myofibroblastoma; a case report and review of the literature. Pathol Oncol Res. 2009;15(2):297–300. doi: 10.1007/s12253-008-9122-0. [DOI] [PubMed] [Google Scholar]
- 18.Silvestre CF, Tavares JA, López-Presa D, dos Santos VR, Rocha J, João Bugalho M. Cervical lymph node schwannoma – an unexpected diagnosis. Clin Pathol. 2019;12:2632010X19829239–2632010X19829239. doi: 10.1177/2632010X19829239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agaimy A, Haller F. CTNNB1 (β-catenin)-altered neoplasia: a review focusing on soft tissue neoplasms and parenchymal lesions of uncertain histogenesis. Adv Anat Pathol. 2016;23(1):1–12. doi: 10.1097/PAP.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 20.Piña-Oviedo S, del Valle L, Baquera-Heredia J, Ortiz-Hidalgo C. Immunohistochemical characterization of Renaut bodies in superficial digital nerves: further evidence supporting their perineurial cell origin. J Peripher Nerv Syst. 2009;14(1):22–26. doi: 10.1111/j.1529-8027.2009.00202.x. [DOI] [PubMed] [Google Scholar]




