α-Glucosidase inhibitory activity of divalent metal complexes bearing hydrazide ligands.
| Entry | Compounds | Structure number | IC50 (μM) | Ref. |
|---|---|---|---|---|
| 1 |
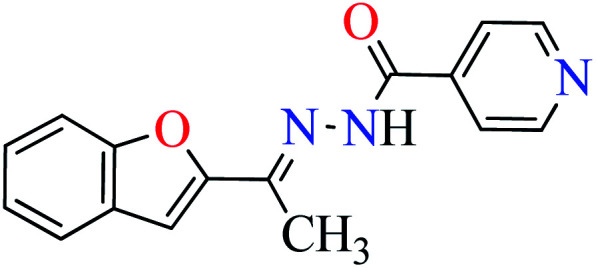
|
L1 | NA | 53 a |
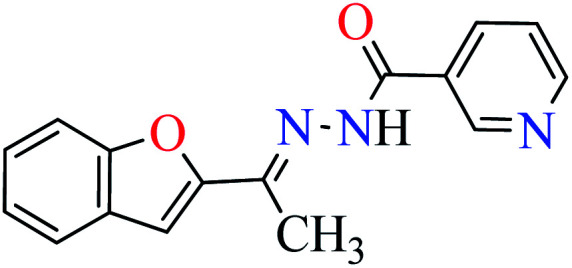
|
L2 | NA | ||
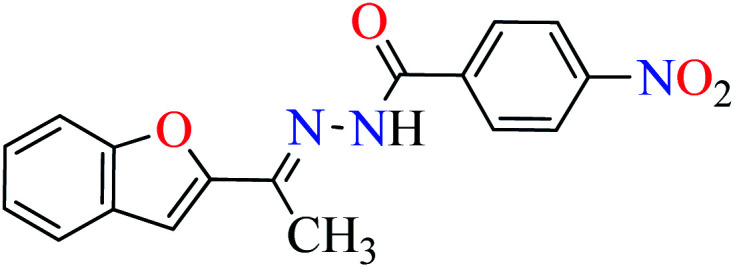
|
L3 | 47.51 | ||
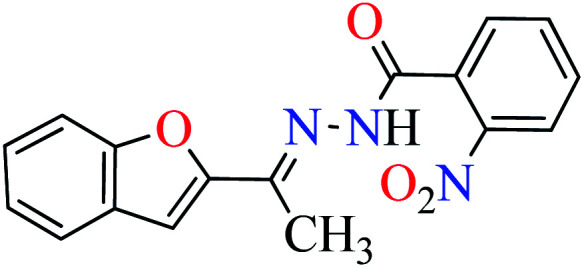
|
L4 | NA | ||
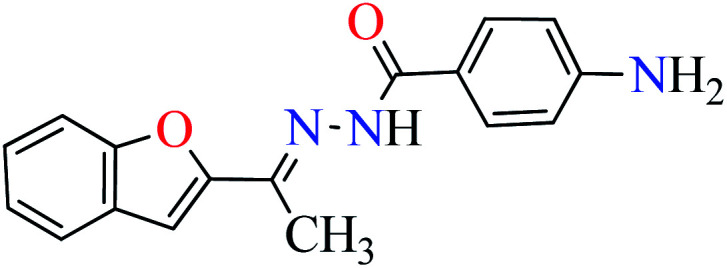
|
L5 | NA | ||
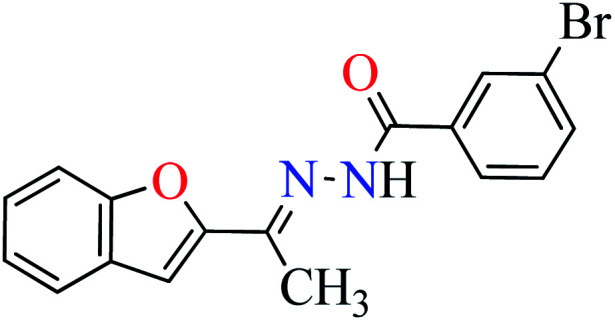
|
L6 | 396.35 | ||
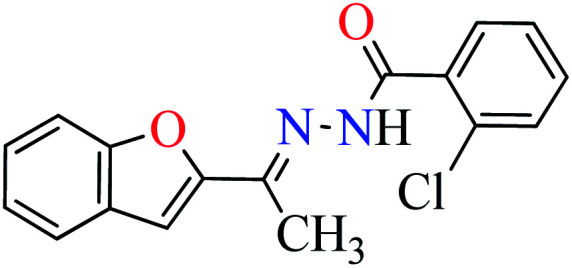
|
L7 | NA | ||
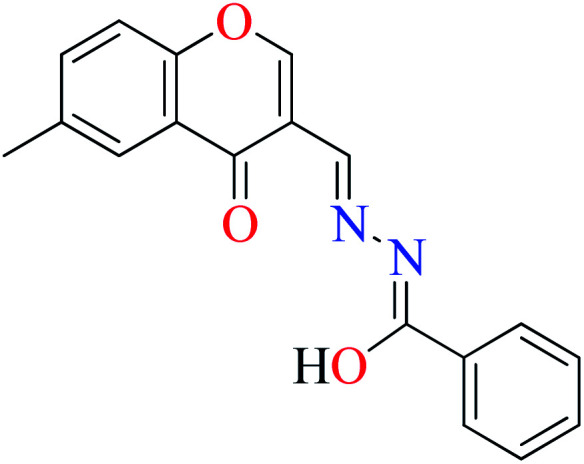
|
L8 | 240 | 54 b | |
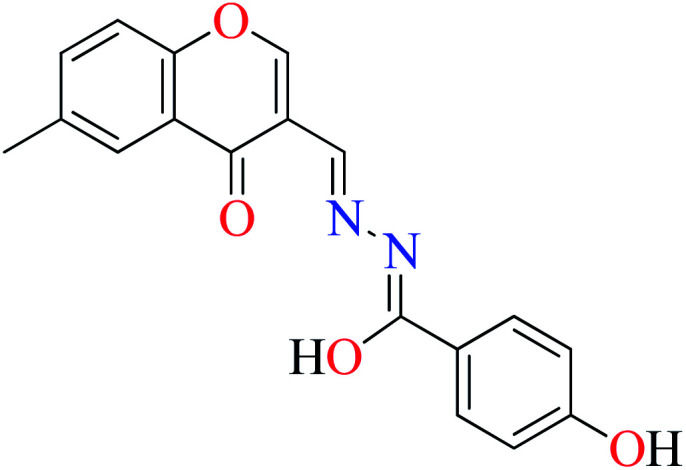
|
L9 | 230 | ||
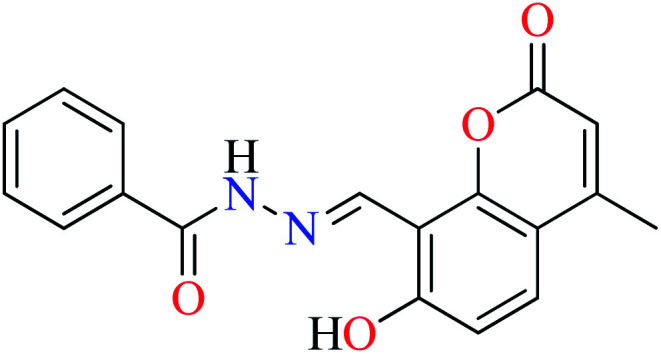
|
L10 | Not reported clearly | 55 b | |
| 2 | 53 a | |||
| 2a |
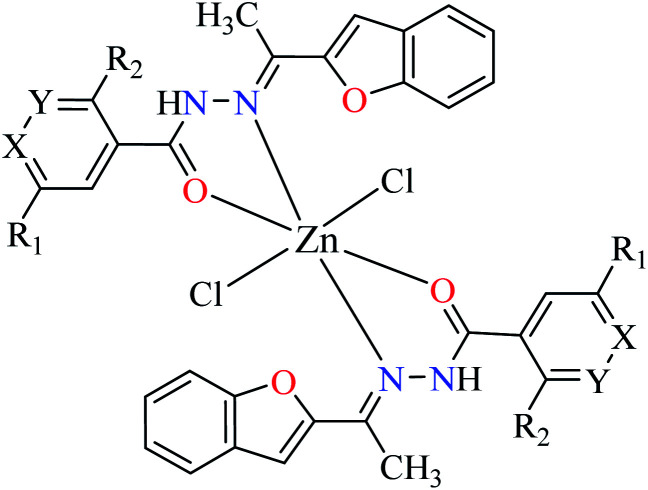
|
|||
| 1 = [Zn(L1)2]Cl2 | 1 X = N; Y = C; R1 = H; R2 = H | 101.29 | ||
| 2 = [Zn(L2)2]Cl2 | 2 X = C; Y = N; R1 = H; R2 = H | 56.27 | ||
| 3 = [Zn(L3)2]Cl2 | 3 X = C–NO2; Y = C; R1 = H; R2 = H | 27.71 | ||
| 4 = [Zn(L4)2]Cl2 | 4 X = C; Y = C; R1 = H; R2 = NO2 | 97.26 | ||
| 5 = [Zn(L5)2]Cl2 | 5 X = C; Y = C–NH2; R1 = H; R2 = H | NA | ||
| 6 = [Zn(L6)2]Cl2 | 6 X = C; Y = C; R1 = Br; R2 = H | 121.19 | ||
| 2b |
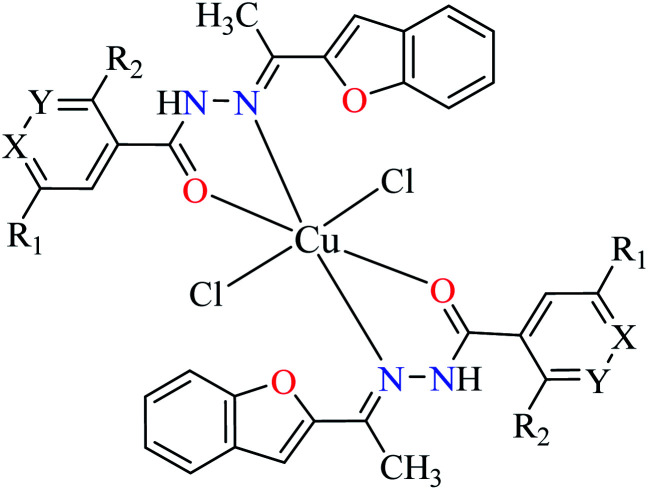
|
|||
| 9 = [Cu(L1)2]Cl2 | 9 X = N; Y = C; R1 = H; R2 = H | >500 | ||
| 10 = [Cu(L2)2]Cl2 | 10 X = C; Y = N; R1 = H; R2 = H | 17.73 | ||
| 11 = [Cu(L3)2]Cl2 | 11 X = C–NO2; Y = C; R1 = H; R2 = H | 1.15 | ||
| 12 = [Cu(L4)2]Cl2 | 12 X = C; Y = C; R1 = H; R2 = NO2 | 18.91 | ||
| 13 = [Cu(L5)2]Cl2 | 13 X = C; Y = C–NH2; R1 = H; R2 = H | 0.15 | ||
| 14 = [Cu(L6)2]Cl2 | 14 X = C; Y = C; R1 = Br; R2 = H | 0.21 | ||
| 15 = [Cu(L7)2]Cl2 | 15 X = C; Y = C; R1 = H; R2 = Cl | NA | ||
| 2c |
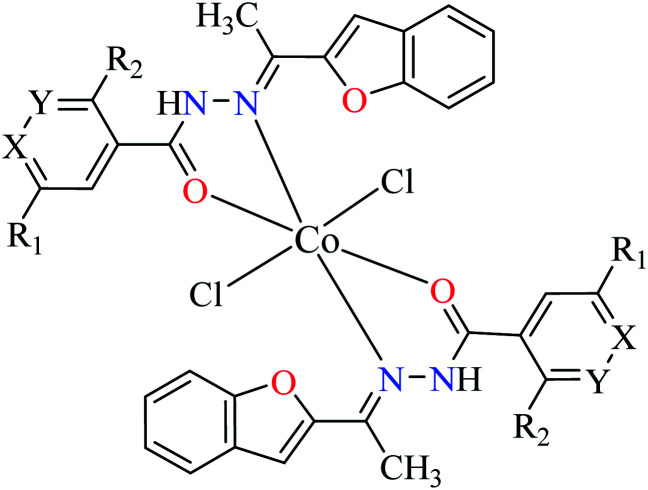
|
|||
| 22 = [Co(L1)2]Cl2 | 22 X = N; Y = C; R1 = H; R2 = H | NA | ||
| 23 = [Co(L2)2]Cl2 | 23 X = C; Y = N; R1 = H; R2 = H | 66.48 | ||
| 24 = [Co(L3)2]Cl2 | 24 X = C–NO2; Y = C; R1 = H; R2 = H | 153.23 | ||
| 25 = [Co(L4)2]Cl2 | 25 X = C; Y = C; R1 = H; R2 = NO2 | 96.95 | ||
| 26 = [Co(L5)2]Cl2 | 26 X = C; Y = C–NH2; R1 = H; R2 = H | NA | ||
| 27 = [Co(L6)2]Cl2 | 27 X = C; Y = C; R1 = Br; R2 = H | 213.30 | ||
| 28 = [Co(L7)2]Cl2 | 28 X = C; Y = C; R1 = H; R2 = Cl | NA | ||
| 2d |
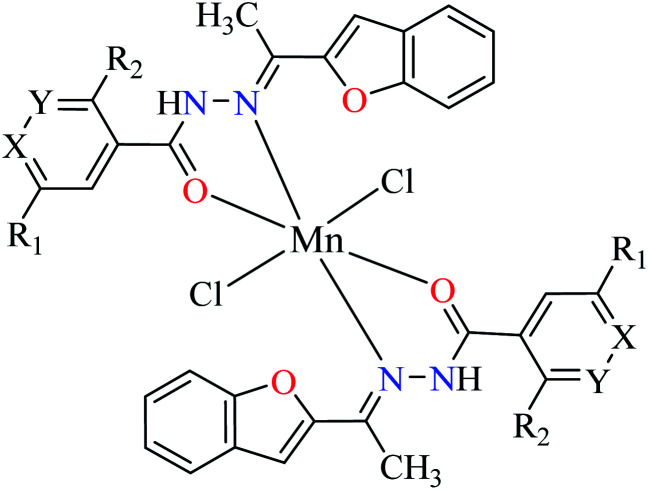
|
|||
| 30 = [Mn(L1)2]Cl2 | 30 X = N; Y = C; R1 = H; R2 = H | 45.63 | ||
| 31 = [Mn(L2)2]Cl2 | 31 X = C; Y = N; R1 = H; R2 = H | 143.21 | ||
| 32 = [Mn(L3)2]Cl2 | 32 X = C–NO2; Y = C; R1 = H; R2 = H | 345.62 | ||
| 33 = [Mn(L4)2]Cl2 | 33 X = C; Y = C; R1 = H; R2 = NO2 | NA | ||
| 34 = [Mn(L5)2]Cl2 | 34 X = C; Y = C–NH2; R1 = H; R2 = H | NA | ||
| 35 = [Mn(L7)2]Cl2 | 35 X = C; Y = C; R1 = H; R2 = Cl | 457.28 | ||
| 3 | 54 b | |||
| 3a |
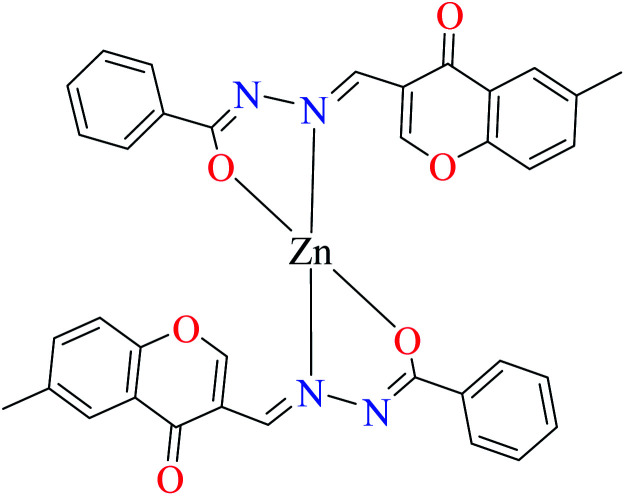
|
|||
| 7 = [Zn(L8)2]·2H2O | 7 | 180 | ||
| 3b |
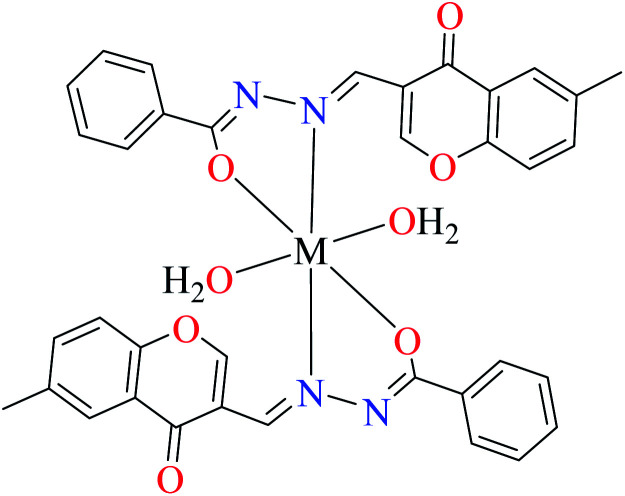
|
|||
| M(ii) = Cu, Ni | ||||
| 16 = [Cu(L8)2(OH2)2]·H2O | 16 | 140 | ||
| 19 = [Ni(L8)2(OH2)2]·H2O | 19 | 200 | ||
| 3c |
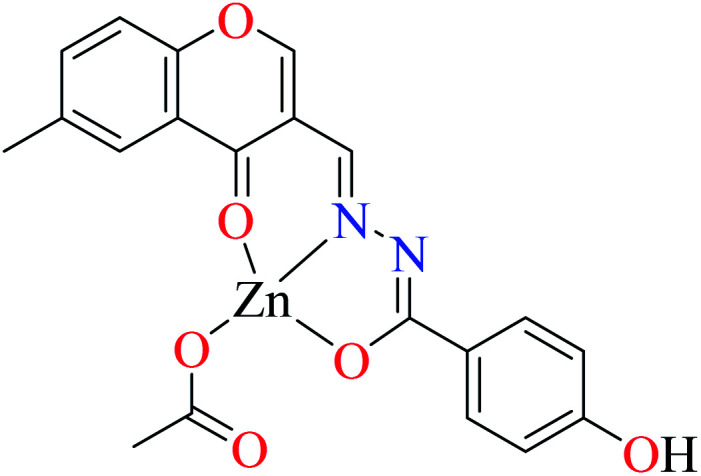
|
|||
| 8 = [Zn(L9)CH3COO]·H2O | 8 | 190 | ||
| 3d |
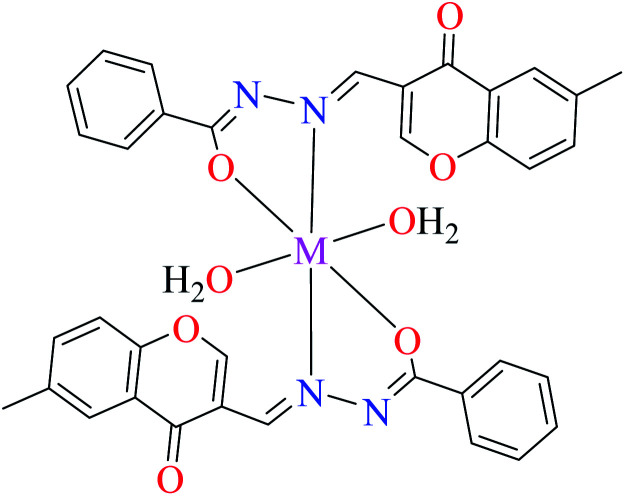
|
|||
| M(ii) = Cu, Ni | ||||
| 17 = [Cu(L9)2(OH2)2]·H2O | 17 | 170 | ||
| 20 = [Ni(L9)2(OH2)2]·H2O | 20 | 230 | ||
| 4 |
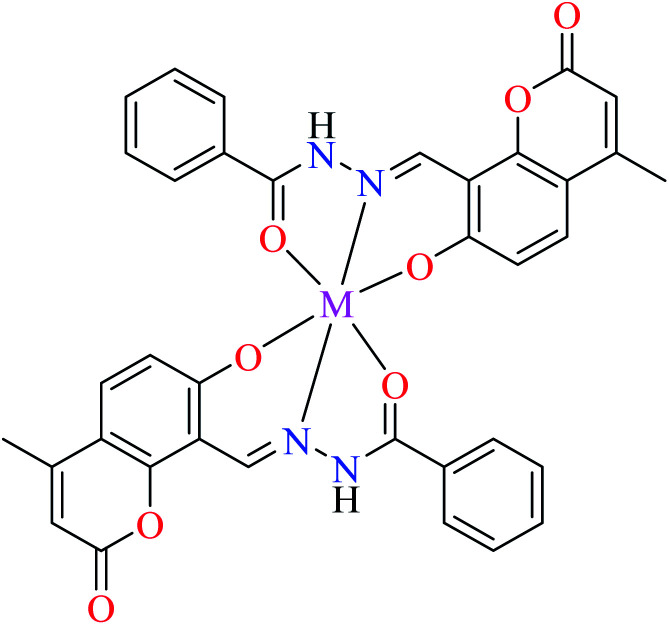
|
55 b | ||
| M(ii) = Cu, Ni, Co, Mn | ||||
| 18 = [Cu(L10)2] | 18 | Not reported clearly | ||
| 21 = [Ni(L10)2] | 21 | Not reported clearly | ||
| 29 = [Co(L10)2] | 29 | Not reported clearly | ||
| 36 = [Mn(L10)2] | 36 | Not reported clearly |
Acarbose as the reference drug (IC50 = 378.25 μM).
Acarbose as the reference drug (IC50 = 99 μM).
