Abstract
Objectives
To evaluate root resorption of lower incisors and canines quantitatively in a group of patients who underwent orthodontic treatment with piezocision and/or a collagen reinforcement technique with a fully resorbable three-dimensional (3D) collagen xenograft matrix compared with a control group.
Materials and Methods
The study sample of this secondary analysis consisted of 32 periodontally healthy patients with angle Class I malocclusion or mild Class II or III malocclusion and moderate irregularity index scores who underwent orthodontic treatment and had before (T0) and after treatment (T1) cone-beam computed tomography scans. Root resorption of lower incisors and canines was assessed quantitatively in the following four groups: the control group received orthodontic treatment without piezocision, experimental group 1 received orthodontic treatment with piezocision, experimental group 2 received orthodontic treatment with piezocision and a 3D collagen matrix, and experimental group 3 received orthodontic treatment with a 3D collagen matrix.
Results
An overall statistically significant decrease in root length from T0 to T1 for all groups was observed (P < .05). However, there was no significant difference among the groups in the amount of root length decrease from T0 to T1.
Conclusions
Orthodontic treatment combined with piezocision does not increase the risk of root resorption of lower incisors and canines when compared with orthodontic treatment without acceleration techniques. More studies with larger samples should be undertaken to confirm these results.
Keywords: Root resorption, Tooth resorption, Piezocision, Corticotomies
INTRODUCTION
Root resorption is the loss of the organic and inorganic component of hard root tissues, such as dentin and cementum, through the continued action of osteoclastic cells1 and can be a result of orthodontic tooth movement.2,3 Orthodontic-induced inflammatory root resorption, a biomechanical phenomenon, is an unwanted risk of orthodontics.4 Therefore, it is important to determine which orthodontic treatment factors contribute to root resorption to minimize harmful effects and reduce the incidence of resorption.5,6 Some of these factors include treatment time and magnitude of tooth displacement, among others.
To accelerate tooth movement, different surgical techniques have been developed for cortical stimulation.7,8 The piezocision technique uses a cutting instrument for decortication without the need to elevate a full thickness flap,9 meaning it is more conservative and less invasive than the traditional corticotomy technique.9 The accelerating impact of corticotomy is attributed to the so-called regional acceleratory phenomenon.7 In addition, corticotomies can stimulate the expression of inflammatory markers and cytokines that lead to increased osteoclast activity.2,3,10,11 Contemporary management with grafts and collagen tissue provides possibilities for strengthening the periodontal phenotype.12
Current evidence supports orthodontic treatment with reduced treatment times,13,14 which decrease root resorption risks, decalcification and caries, and periodontal alterations while enhancing the personal commitment to patients.15,16 Hence, it is crucial to determine the comprehensive safety of techniques for accelerating treatment time, especially regarding the risk of producing root resorption. It would also be possible to establish whether this type of approach could provide a protective factor against root resorption.9 Therefore, the present study aimed to evaluate changes in tooth length of the lower incisors and canines in a group of patients who underwent orthodontic treatment with piezocision, and/or collagen reinforcement techniques using a collagen matrix with high biocompatibility, compared with a control group.
MATERIALS AND METHODS
This study was a secondary analysis of a controlled clinical trial enrolled at ClinicalTrials.gov (identification no. NCT02866929; unpublished results). The cone-beam computed tomography (CBCT) scans prescribed during the clinical trial were part of the medical records at Universidad Corporación para Estudios en la Salud (CES) in Medellin, for which the guarantees and permissions of use of the institution were obtained. The Institutional Committee for Human Research Ethics approved the protocol of this project through act number 86 in session on October 13, 2015.
In the present study, CBCT scans of 32 consecutive patients prospectively collected in the previous clinical trial were analyzed before (T0) and after treatment (T1). Periodontal parameters for all patients were collected before and after orthodontic therapy with a standardized protocol using a computerized periodontal probing and comprehensive charting system (Florida probe Corp, Florida, United States). All patients were periodontally healthy with at least 2 mm of keratinized gingiva, angle Class I or mild angle Class II or III malocclusion, and moderate irregularity according to the Little Irregularity Index17 and who underwent orthodontic treatment with a Damon passive self-ligating bracket system (Ormco Corp, Ontario, Canada). The control group consisted of 8 patients who underwent orthodontic treatment only. Experimental group 1 consisted of 7 patients treated with piezocision. Experimental group 2 consisted of 9 patients treated with piezocision and a collagen matrix with high biocompatibility (Geistlich Mucograft®, Geistlich Pharma AG, Wolhusen, Switzerland) in the lower interincisive zone. Experimental group 3 consisted of 8 patients who underwent orthodontic treatment and anteroinferior collagen reinforcement with a collagen matrix with high biocompatibility in the lower interincisive zone. The observations were restricted to this area based on the inclusion criteria focused on sites defined as areas with thin periodontal phenotype and dental crowding.
The modified piezocision technique used in the trial was carried out using a Satelec Acteon Piezotome ultrasonic surgery unit (Acteon, Mount Laurel, N.J.) by two expert periodontists. The procedure was performed after positioning the orthodontic appliances and following the protocol described by Dibart et al.18 The available CBCT scans facilitated/assisted in the correlation of the crown anatomy with the root location and orientation. Corticotomies were limited in the lower anterior teeth to the interradicular spaces between the central incisors and between the lateral incisors and canines. Under local anesthesia, vertical and inter-radicular gingival incisions were made in the buccal surface of the maxillary and mandibular arches, starting 2 to 3 mm below the interdental papilla and with sufficient depth to the periosteum to allow the scalpel to reach the alveolar bone. These incisions were kept as small as possible. Subsequently, through these incisions, a piezoelectric scalpel (piezotome) was used to make cuts in the bone deep enough to pierce the alveolar cortex. Once the corticotomy was accomplished, in the area where the graft was performed (lower interincisive zone), tunneling and connection with the gingival margin was carried out. The collagen matrix with high biocompatibility was positioned to the tissue and sutured using resorbable suture 5-0, except in the areas where no tunneling was performed.
CBCT scans were obtained using the Veraviewepocs 3D R100 (J Morita Corp, Tokyo, Japan) according to the following acquisition protocol: 100 × 80 mm field of view; 0.16 mm3 voxel size, 90 kVp, 3 to 5 mA, and 9.3 seconds.
Root resorption was assessed by one observer (Juan Fernando Aristizábal) using the following two open-source software applications: ITK-Snap version 2.4.0 (http://www.itksnap.org) and 3D Slicer version 4.10.1 (https://www.slicer.org). To estimate the root resorption that occurred between T0 and T1, the following procedures were carried out on the CBCT scans:
Digital Imaging and Communication On Medicine (DICOM) files of the CBCT scans were converted into “gipl.gz” files using the ITK-Snap software.
Three-dimensional (3D) volumetric label maps (segmentation) of the T0 mandibles from the “gipl.gz” files were constructed.
3D surface models were generated from 3D volumetric label maps of each mandible using the 3D Slicer software. A standardized common orientation of T0 3D surface models was performed using the transforms tool. The matrix generated from the orientation was applied to the T0 scans and segmentations. Approximation and voxel-based registration (mandibular regional superimposition) of T1 CBCT scans in relation to the oriented T0 CBCT file was achieved using the nongrowing registration module.19
3D volumetric label maps (segmentation) and 3D surface models of the T1 mandibles from the registered T1 scans were constructed as described for T0.
A total of twelve 3D dots were placed on the T0 and T1 segmentations for prelabeling. The dots were located at the lower incisors (the most apical part of the root and at the central point of the incisal edge) and lower canines (the most apical part of the root and at the central point of the tip). After prelabeling, T0 and T1 mandibular 3D surface models were generated (vtk files).20
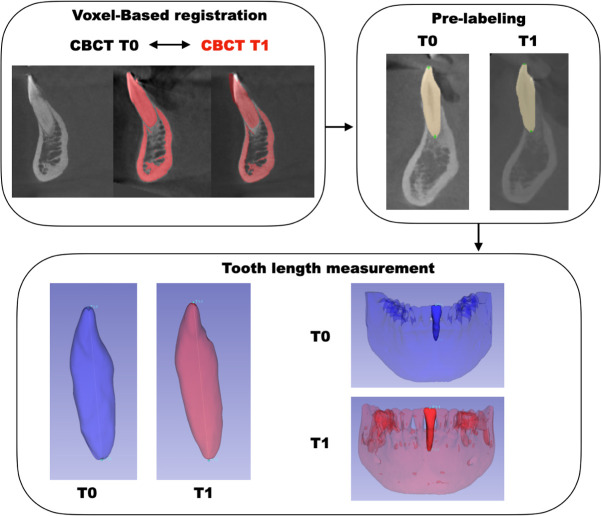
Measurements were performed using the “Quantification of 3D Components” tool in Slicer. Fiducial landmarks were placed following the prelabeled 3D dots in the segmentation made to determine the tooth length of the lower incisors and canines (Figure 1).
Figure 1.
Flowchart: assessment of tooth length.
Statistical Analysis
To test intraobserver repeatability of the methodology, the entire protocol was repeated in five randomly selected CBCT scans by one observer, and the Intraclass correlation coefficient (ICC) was calculated. The Kolmogorov-Smirnov test was used to determine normality of the data distribution. The outcome data were not normally distributed; therefore, nonparametric tests were used. The Wilcoxon test was used to compare intragroup changes from T0 to T1. The Kruskal-Wallis test was used to compare the differences at baseline and in the mean changes from T0 to T1 among the four groups. All statistical analyses were conducted using SPSS Statistics for Mac version 25.0 (IBM, Armonk, N.Y.).
RESULTS
The outcome variable had excellent repeatability. The intraobserver ICC was 0.879. Of the 32 patients, 24 were men (75%) and the mean age was 26.9 ± 5.8 years (range, 19–38 years). A total of 192 roots were analyzed. In five patients, one of the six teeth had buccal and lingual roots. In those cases, only the buccal roots were measured.
The outcome variables of the patients at T0 are summarized in Table 1. Overall, root length significantly decreased from T0 to T1 in all groups (P < .05). The mean treatment time for the control group was 456.63 days; for experimental group 1, 409.43 days; for experimental group 2, 344.33 days; and for experimental group 3, 385.25 days.
Table 1.
Comparison of Age, the Little Irregularity Index, Treatment Time, and Root Length for Groups at T0a
| Variables |
Control, n = 8 |
Experimental Group 1, n = 7 |
Experimental Group 2, n = 9 |
Experimental Group 3, n = 8 |
P Value, Kruskal-Wallis Test |
| Age, years | 24 (6.07) | 21.29 (4.5) | 29.11 (6.45) | 27.5 (3.34) | .037* |
| Little Irregularity Index, mm | 10.01 (2.41) | 10.67 (1.54) | 9.44 (1.56) | 11.95 (3.93) | .458 |
| Treatment time, days | 456.63 (86.21) | 409.43 (146.45) | 344.33 (72.48) | 385.25 (106.76) | .153 |
| Root length 33 mm | 26.16 (2.91) | 26.64 (3.40) | 25.2 (1.23) | 25.6 (0.91) | .948 |
| Root length 32 mm | 22.66 (1.57) | 23.32 (1.98) | 22.16 (1.81) | 22.38 (1.55) | .701 |
| Root length 31 mm | 20.92 (1.62) | 21.36 (1.90) | 20 (1.85) | 21.12 (1.47) | .484 |
| Root length 41 mm | 20.87 (1.67) | 21.45 (1.41) | 20.07 (1.50) | 20.91 (1.01) | .412 |
| Root length 42 mm | 22.77 (2.46) | 23.48 (1.45) | 22.1 (1.65) | 22.8 (1.84) | .456 |
| Root length 43 mm | 26.14 (2.81) | 25.62 (3.67) | 25.19 (1.96) | 26.02 (1.49) | .846 |
Data are provided as mean (standard deviation [SD]).
P < .05.
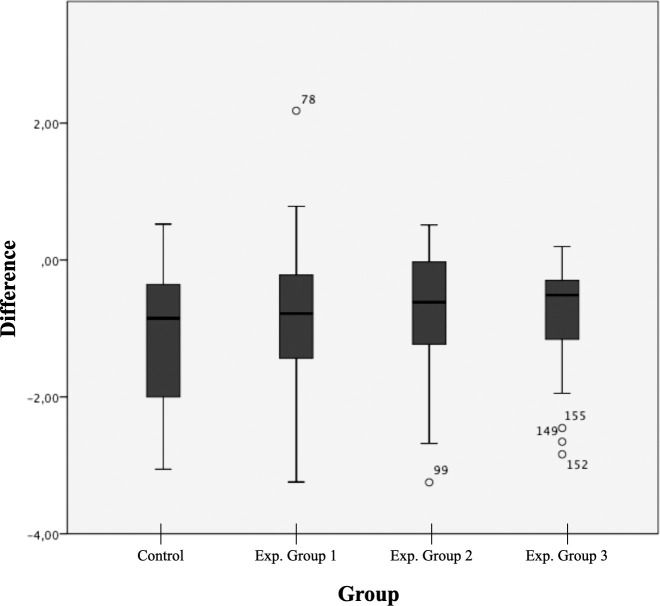
When comparing the changes in root length from T0 to T1 among groups, no statistically significant differences were found (P > .05; Table 2). Experimental group 2 showed the lowest average change in root length from T0 to T1 (−0.7 ± 0.86 mm), followed by experimental groups 3 (−0.81 ± 0.74 mm) and 1 (−0.83 ± 0.99 mm). The control group showed the highest overall loss in root length from T0 to T1 (−1.07 ± 0.95 mm), as shown in Figure 2. The boxplot illustrates that there were no significant differences among the groups in the amounts of root length shortening from T0 to T1 and that there was a high dispersion of the data.
Table 2.
Comparison of Each Root Length at T0 and T1 and the Difference by Time (T1–T0) for Groups
| Root Length, mm |
Control, n = 8 |
Experimental Group 1, Piezocision, n = 7 |
||||||||||
| T0 | T1 | T1–T0 | 95% CIa |
Wilcoxon Signed Rank Test | T0 | T1 | T1–T0 | 95% CIa |
Wilcoxon Signed Rank Test | |||
| Mean (SD) |
Mean (SD) |
Mean (SD) |
Lower |
Upper |
P Value |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Lower |
Upper |
P Value |
|
| 33 | 26.16 (2.91) | 25.36 (2.97) | −0.80 (1.15) | −0.16 | 1.76 | .093 | 26.64 (3.40) | 26.23 (3.54) | −0.42 (0.34) | 0.10 | 0.73 | .018* |
| 32 | 22.66 (1.57) | 21.61 (1.97) | −1.04 (0.82) | 0.36 | 1.73 | .017* | 23.32 (1.98) | 22.54 (1.79) | −0.78 (0.78) | 0.06 | 1.49 | .043* |
| 31 | 20.92 (1.62) | 19.6 (1.75) | −1.32 (0.99) | 0.49 | 2.15 | .012* | 21.36 (1.90) | 20.11 (1.97) | −1.25 (0.94) | 0.38 | 2.11 | .018* |
| 41 | 20.87 (1.67) | 19.92 (2.27) | −0.94 (0.90) | 0.19 | 1.69 | .025* | 21.45 (1.41) | 20.19 (1.50) | −1.26 (0.99 | 0.35 | 2.17 | .018* |
| 42 | 22.77 (2.46) | 21.29 (2.39) | −1.48 (0.92) | 0.71 | 2.25 | .012* | 23.48 (1.45) | 22.06 (1.52) | −1.42 (0.76) | 0.72 | 2.12 | .018* |
| 43 | 26.14 (2.81) | 25.30 (2.75) | −0.84 (1.06) | −0.04 | 1.73 | .036* | 25.62 (3.67) | 25.74 (3.57) | 0.12 (1.26) | −1.29 | 1.05 | .735 |
CI indicates confidence interval.
P < .05.
Table 2.
Extended
| Experimental Group 2, Piezocision and Mucograft, n = 9 |
Experimental Group 3, Mucograft, n = 8 |
T1–T0 Group 1 vs 2 vs 3 vs 4 |
||||||||||
| T0 | T1 | T1–T0 | 95% CIa |
Wilcoxon Signed Rank Test | T0 | T1 | T1–T0 | 95% CIa |
Wilcoxon Signed Rank Test | Kruskal-Wallis Test | ||
| Mean (SD) |
Mean (SD) |
Mean (SD) |
Lower |
Upper |
P Value |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Lower |
Upper |
P Value |
P Value |
| 25.2 (1.23) | 24.78 (1.33) | −0.42 (0.81) | −0.19 | 1.05 | .314 | 25.6 (0.91) | 25.24 (0.74) | −0.36 (0.34) | 0.07 | 0.64 | .025* | .908 |
| 22.16 (1.81) | 21.37 (1.65) | −0.79 (0.98) | 0.04 | 1.55 | .038* | 22.38 (1.55) | 21.55 (1.25) | −0.84 (0.86) | 0.12 | 1.55 | .012* | .826 |
| 20 (1.85) | 18.94 (1.26) | −1.06 (0.90) | 0.37 | 1.75 | .008* | 21.12 (1.47) | 20.01 (1.38) | −1.11 (0.68) | 0.54 | 1.67 | .012* | .936 |
| 20.07 (1.50) | 19.32 (1.05) | −0.75 (0.92) | 0.03 | 1.45 | .028* | 20.91 (1.01) | 19.99 (1.26) | −0.92 (0.64) | 0.38 | 1.46 | .012* | .675 |
| 22.1 (1.65) | 21.15 (1.61) | −0.95 (0.91) | 0.25 | 1.65 | .028* | 22.8 (1.84) | 21.70 (1.56) | −1.10 (1) | 0.27 | 1.93 | .012* | .603 |
| 25.19 (1.96) | 24.96 (1.91) | −0.23 (0.49) | −0.14 | 0.61 | .173 | 26.02 (1.49) | 25.51 (1.65) | −0.51 (0.65) | −0.03 | 1.06 | .025* | .241 |
Figure 2.
Boxplot displaying differences among the following four groups analyzed: control group and experimental groups 1 to 3. Exp indicates experimental.
DISCUSSION
Root resorption is the loss of the organic and inorganic component of hard root tissues, including dentin and cementum, through the continued action of osteoclastic cells.1 The main objective of this study was to evaluate and compare root resorption in a group of patients undergoing orthodontic treatment with piezocision and collagen reinforcement techniques with a collagen matrix with high biocompatibility. The results showed no significant differences among the four groups compared, suggesting that orthodontic treatment combined with piezocision in the region of the mandibular anterior teeth did not increase the risk of root resorption for those teeth compared with performing orthodontic treatment without the acceleration techniques.
Root resorption can be associated with orthodontic movement. This can occur when the forces exerted on the tooth, which are transmitted to the root, exceed the repair capacity of its tissues.21,22 The dynamics of mechanotransduction allow the biological components to be activated from force, with the final consequence of osteoclast proliferation in the compression zone, followed by resorption of bone and, occasionally, root cementum.23 The current study showed changes in root cementum remodeling in the treated groups. However, in no case were the lesions greater than those reported in the literature as normal findings.2,9,11,24 There were no significant differences in the amount of root length decrease observed among the four groups evaluated in the current study, which was consistent with other studies, such as the study by Charavet et al.,25 which also reported no increase in root resorption associated with the use of the piezocision technique.
The detail and quality of the records used in the current study afforded a precise method by which to detect loss of root cementum. Traditional diagnostic tools previously used to evaluate root resorption were of limited value because they were not precise. Typically, the radiographic images used were two-dimensional, causing difficulties for superimposition of sequential images and, therefore, inaccuracy in the analysis.26,27 CBCT allowed for better detection of root resorption than two-dimensional radiographs, providing excellent diagnostic monitoring of root length changes.2,24 Root resorption has been studied previously for various orthodontic treatment techniques, and variable results have been reported.9,28,29 Although the force levels applied were theoretically lower using self-ligating brackets, no differences in root resorption were previously reported compared with conventional appliance treatment.30–33 In the current study, the alterations in the root cementum layer observed in the six lower anterior teeth evaluated were less than those found in conventional treatment31–33 performed with decortication techniques. Only the six mandibular anterior teeth were evaluated in the current study due to the inclusion criteria of the original clinical trial for which the data were collected.
Root resorption has previously been associated with cases treated using accelerated tooth movement techniques. Patterson et al.34 evaluated 28 bilaterally extracted upper premolars from 14 patients. They used the piezocision technique on one side of the maxilla and conventional orthodontics on the other side. The authors found a significantly greater total amount of root resorption on the side in which the piezocision technique was performed compared with the control side (P = .029). However, tissue damage was a product of an inadequate piezocision technique and not of the expressed movement, thus being an iatrogenic lesion caused by the instrument used.34
Makhoul et al.35 evaluated 144 anterior maxillary teeth of 24 patients treated with conventional appliances in one group and with modified piezocision in the other group. CBCT was used to assess root resorption at two stages (T0, T1). The results at T1 showed statistically significant differences in the control group, whereas in the piezocision group, no statistically significant differences were found between T0 and T1 for all the variables studied.35
In addition, in a study conducted by Machado et al.,36 a group of 27 patients treated without extraction with facilitated corticotomy was compared with a group of 27 patients treated with conventional orthodontics. Using periapical radiographs, the root lengths before treatment were not significantly different (P = .11) compared with after treatment. The authors concluded that, under the conditions of their study, orthodontic treatment without extraction facilitated by corticotomy resulted in less root resorption.36 The findings of the current study were that root lengths decreased in all treated groups, with and without piezocision, corresponding to normal treatment remodeling, with amounts lower than those reported in the literature.2,9,11,24
Under the conditions of the present study, it was shown that, in patients treated with passive self-ligating systems, with or without piezocision as an acceleration technique, in mild Class I, II, and III treatments and with mild to moderate crowding treated without extractions, there were no differences among the groups in terms of the amount of root shortening observed. However, more studies with larger sample sizes should be conducted to further validate these results.
CONCLUSIONS
The results of the current study show that orthodontic treatment combined with piezocision does not increase the risk of root resorption to mandibular incisors and canines when compared with orthodontic treatment without acceleration techniques. However, considering the limitations of the present study in terms of sample size, there is a need for more studies with greater sample sizes to further validate the results obtained.
ACKNOWLEDGMENTS
Disclosure The authors declare that there is no conflict of interest regarding the publication of this article. The authors had no financial support for the development of this study.
REFERENCES
- 1.Darcey J, Qualtrough A. Root resorption: simplifying diagnosis and improving outcomes. Prim Dent J . 2016;5(2):36–45. doi: 10.1308/205016816819304222. [DOI] [PubMed] [Google Scholar]
- 2.Weltman B, Vig KW, Fields HW, et al. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop . 2010;137(4):462–476. doi: 10.1016/j.ajodo.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Fuss Z, Tsesis I, Lin S. Root resorption—diagnosis, classification and treatment choices based on stimulation factors. Dent Traumatol . 2003;19(4):175–182. doi: 10.1034/j.1600-9657.2003.00192.x. [DOI] [PubMed] [Google Scholar]
- 4.Abuabara A. Biomechanical aspects of external root resorption in orthodontic therapy. Med Oral Patol Oral Cir Bucal . 2007;12(8):E610–E613. [PubMed] [Google Scholar]
- 5.Roscoe MG, Meira JB, Cattaneo PM. Association of orthodontic force system and root resorption: a systematic review. Am J Orthod Dentofacial Orthop . 2015;147(5):610–626. doi: 10.1016/j.ajodo.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Gupta V, Dhaliwal YS, Dhaliwal A. Root resorption associated with orthodontic tooth movement: a review. Dent J Adv Stud . 2016;4:8–14. [Google Scholar]
- 7.Aristizábal JF, Bellaiza W, Ortiz MA, et al. Clinical and systemic effects of periodontally accelerated osteogenic orthodontics: a pilot study. Int J Odontostomat . 2016;10:119–127. [Google Scholar]
- 8.Aristizábal JF. Accelerated orthodontics and express transit orthodontics (ETO)®, a contemporary concept of high efficiency. CES Odontol . 2014;27:56–73. [Google Scholar]
- 9.Abbas NH, Sabet NE, Hassan IT. Evaluation of corticotomy-facilitated orthodontics and piezocision in rapid canine retraction. Am J Orthod Dentofacial Orthop . 2016;149(4):473–480. doi: 10.1016/j.ajodo.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Charavet C, Lecloux G, Jackers N, et al. Piezocision-assisted orthodontic treatment using CAD/CAM customized orthodontic appliances: a randomized controlled trial in adults. Eur J Orthod . 2019;41(5):495–501. doi: 10.1093/ejo/cjy082. [DOI] [PubMed] [Google Scholar]
- 11.Alfawal AM, Hajeer MY, Ajaj MA, et al. Effectiveness of minimally invasive surgical procedures in the acceleration of tooth movement: a systematic review and meta-analysis. Prog Orthod . 2016;17(1):33. doi: 10.1186/s40510-016-0146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson DJ, Wilcko MT, Wilcko WM, et al. Scope of treatment with periodontally accelerated osteogenic orthodontics therapy. Semin Orthod . 2015;21:176–186. [Google Scholar]
- 13.Yavuz MC, Sunar O, Buyuk SK, et al. Comparison of piezocision and discision methods in orthodontic treatment. Prog Orthod . 2018;19(1):44. doi: 10.1186/s40510-018-0244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linjawi AI, Abushal AM, Al-Zahrani AM, et al. Patients' perceptions to reduced orthodontic treatment time in Saudi Arabia. Patient Prefer Adherence . 2019;13:1973–1981. doi: 10.2147/PPA.S222181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Jacox LA, Little SH, et al. Orthodontic tooth movement: the biology and clinical implications. Kaohsiung J Med Sci . 2018;34(4):207–214. doi: 10.1016/j.kjms.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Fleming PS. Accelerating orthodontic tooth movement using surgical and non-surgical approaches. Evid Based Dent . 2014;15(4):114–115. doi: 10.1038/sj.ebd.6401063. [DOI] [PubMed] [Google Scholar]
- 17.Little RM. The irregularity index: a quantitative score of mandibular anterior alignment. Am J Orthod . 1975;68(5):554–563. doi: 10.1016/0002-9416(75)90086-x. [DOI] [PubMed] [Google Scholar]
- 18.Dibart S, Sebaoun JD, Surmenian J. Piezocision: a minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Compend Contin Educ Dent . 2009;30(6):342–350. [PubMed] [Google Scholar]
- 19.Ruellas AC, Yatabe MS, Souki BQ, et al. 3D mandibular superimposition: comparison of regions of reference for voxel-based registration. PLoS One . 2016;11(6):e0157625. doi: 10.1371/journal.pone.0157625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruellas AC, Huanca Ghislanzoni LT, Gomes MR, et al. Comparison and reproducibility of 2 regions of reference for maxillary regional registration with cone-beam computed tomography. Am J Orthod Dentofac Orthop . 2016;149(4):533–542. doi: 10.1016/j.ajodo.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi J, Xiao J, Li Y, et al. Efficacy of piezocision on accelerating orthodontic tooth movement: a systematic review. Angle Orthod . 2017;87(4):491–498. doi: 10.2319/01191-751.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker RJ, Harris EF. Directions of orthodontic tooth movements associated with external apical root resorption of the maxillary central incisor. Am J Orthod Dentofacial Orthop . 1998;114(6):677–683. doi: 10.1016/s0889-5406(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 23.Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: part 1. Literature review. Am J Orthod Dentofacial Orthop . 1993;103(1):62–66. doi: 10.1016/0889-5406(93)70106-X. [DOI] [PubMed] [Google Scholar]
- 24.Aksakalli S, Calik B, Kara B, et al. Accelerated tooth movement with piezocision and its periodontal-transversal effects in patients with Class II malocclusion. Angle Orthod . 2016;86(1):59–65. doi: 10.2319/012215-49.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charavet C, Lecloux G, Bruwier A, et al. Localized piezoelectric alveolar decortication for orthodontic treatment in adults: a randomized controlled trial. J Dent Res . 2016;95(9):1003–1009. doi: 10.1177/0022034516645066. [DOI] [PubMed] [Google Scholar]
- 26.Chan EK, Darendeliler MA. Exploring the third dimension in root resorption. Orthod Craniofac Res . 2004;7(2):64–70. doi: 10.1111/j.1601-6343.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 27.Darcey J, Qualtrough A. Resorption: part 2. Diagnosis and management. Br Dent J . 2013;214(10):493–509. doi: 10.1038/sj.bdj.2013.482. [DOI] [PubMed] [Google Scholar]
- 28.Chan E, Dalci O, Petocz P, et al. Physical properties of root cementum: part 26. Effects of micro-osteoperforations on orthodontic root resorption: a microcomputed tomography study. Am J Orthod Dentofacial Orthop . 2018;153(2):204–213. doi: 10.1016/j.ajodo.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 29.Thind SK, Chatterjee A, Arshad F, et al. A clinical comparative evaluation of periodontally accelerated osteogenic orthodontics with piezo and surgical bur: an interdisciplinary approach. J Indian Soc Periodontol . 2018;22(4):328–333. doi: 10.4103/jisp.jisp_359_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Songra G, Clover M, Atack NE, et al. Comparative assessment of alignment efficiency and space closure of active and passive self-ligating vs conventional appliances in adolescents: a single-center randomized controlled trial. Am J Orthod Dentofacial Orthop . 2014;145:569–578. doi: 10.1016/j.ajodo.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs C, Gebhardt PF, Jacobs V, et al. Root resorption, treatment time and extraction rate during orthodontic treatment with self-ligating and conventional brackets. Head Face Med . 2014;10:2. doi: 10.1186/1746-160X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson K, Lundström F. Orthodontic treatment efficiency with self-ligating and conventional edgewise twin brackets: a prospective randomized clinical trial. Angle Orthod . 2012;82(5):929–934. doi: 10.2319/101911-653.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandis N, Nasika M, Polychronopoulou A, et al. External apical root resorption in patients treated with conventional and self-ligating brackets. Am J Orthod Dentofacial Orthop . 2008;134(5):646–651. doi: 10.1016/j.ajodo.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 34.Patterson BM, Dalci O, Papadopoulou AK, et al. Effect of piezocision on root resorption associated with orthodontic force: a microcomputed tomography study. Am J Orthod Dentofacial Orthop . 2017;151(1):53–62. doi: 10.1016/j.ajodo.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Makhoul F, Jaafo MH, Ajai M. Evaluation of the effectiveness of alveolar corticotomy on reducing orthodontic root resorption. J Indian Dent Assoc . 2014;8:31–40. [Google Scholar]
- 36.Machado I, Ferguson DJ, Wilcko TM, et al. Reabsorción radicular después del tratamiento ortodóncico con o sin corticotomía alveolar. Rev Venez Ortod . 2002;19(1):647–653. [Google Scholar]