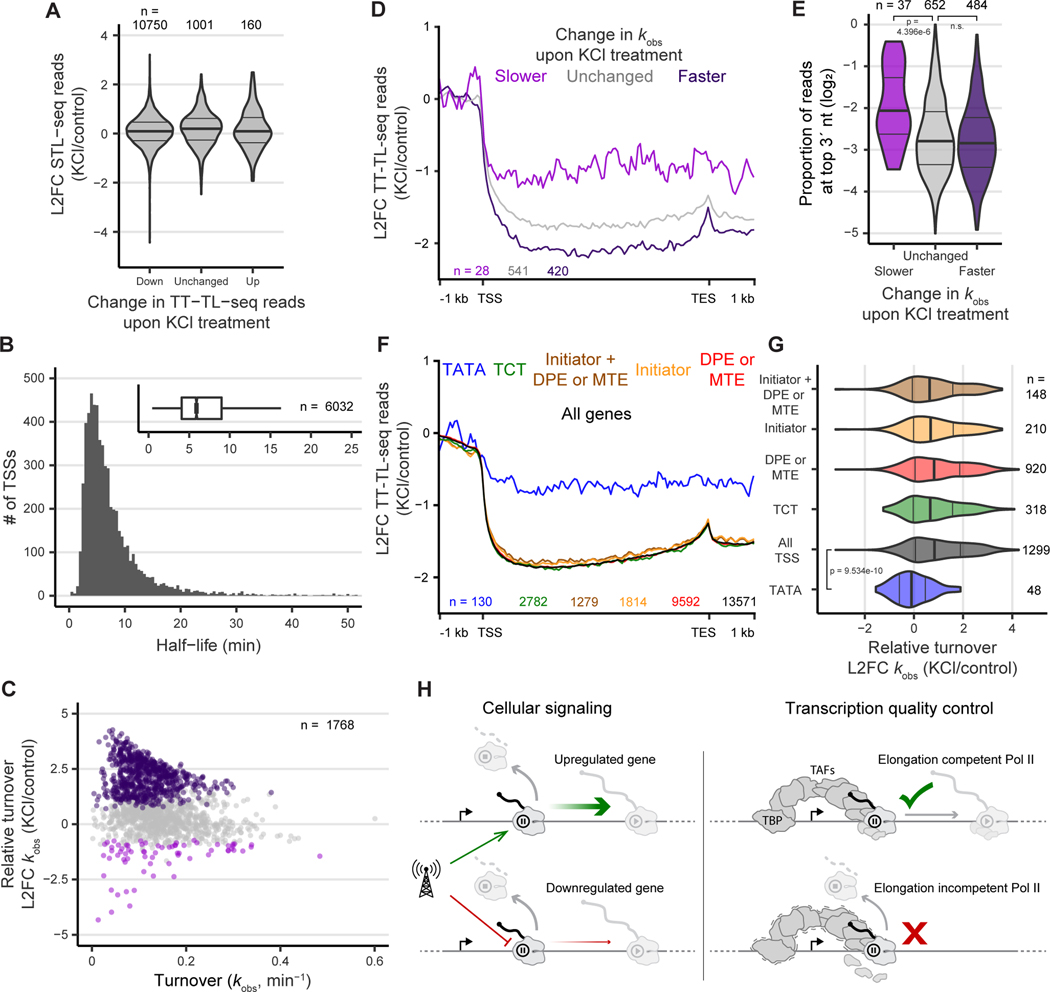
Figure 6. Hyperosmotic stress induces premature termination at TATA-less promoters.
(A) Change STL-seq reads at promoter TSSs grouped by the change in TT-TL-seq signal over the gene body.
(B) Histogram of scRNA half-life estimates made with STL-seq from human 293T cells. The inset boxplot depicts the distribution of all TSSs.
(C) Total observed rate constants of TSSs plotted versus the log2 fold change when cells are exposed to hyperosmotic stress for one hour. Points are colored if the 80% credible interval is entirely greater than log2(1.5) (dark purple) or less than -log2(1.5) (light purple).
(D) Metaplots of the log2 fold change of TT-TL-seq signal upon hyperosmotic stress grouped by the change in turnover of scRNA at the gene’s TSS as determined by STL-seq. Coverage was determined over 50 nt bins before calculating the fold change of each bin.
(E) Distribution of the proportion of reads at promoters with 3´ ends located at the most frequent pause position grouped by the change in scRNA turnover and colored as in D. At each TSS the most common position of the 3´ read end was identified, and the proportion of reads at this position was determined.
(F) Metaplots of the log2 fold change of TT-TL-seq signal upon hyperosmotic stress grouped by the motif content of the associated STL-seq TSS. Coverage was determined over 50 nt bins before calculating the fold change of each bin.
(G) The distribution of the log2 fold change of scRNA turnover rate constants at promoters upon hyperosmotic stress. TSSs are grouped by motif content and colored as in F. Significance was assessed by a two-sided Wilcoxon rank sum test.
(H) Proposed model for the distinct roles of release into elongation and premature termination at the promoter-proximal pause site. To alter gene expression, cells signal for either an increase or decrease in release into elongation (left). Premature termination does not contribute greatly to the response to cellular signaling but acts to evict paused Pol II whose elongation factors do not assemble properly (right). Coordinated binding of TFIID subunits, TBP and TAFs, is important for maturation of an elongation-competent Pol II and significantly stabilizes the mature complex.