Abstract
Background
COVID-19 is associated with depressive psychopathology in survivors. Negative thinking styles are a core feature of major depression, fostering the experience of negative emotions and affects and hampering recovery. This cognitive vulnerability has been observed in medical conditions associated with depression, but never explored in post-COVID depression.
Methods
We studied 729 participants: 362 COVID-19 survivors, 73 inpatients with Major Depressive Disorder (MDD), and 294 healthy participants (HC). Severity of depression was self-rated on the Zung Self-Rating Depression Scale (ZSDS). Neuropsychological bias toward negative emotional stimuli and the negative outlook on the self were tested in a self-description task, yielding latencies and frequencies of attribution of morally tuned elements. Dimensions of negative thinking and depressive cognitive style in evaluation of hypothetical events were measured on the Cognition Questionnaire (CQ).
Results
22.4% COVID survivors self-rated depression above the clinical threshold. Frequencies and latencies of attribution of morally negative elements, and CQ scores, correlated between themselves and predicted ZSDS scores, with post-COVID depressed patients showing intermediate scores between the more severe MDD patients, and non-depressed post-COVID participants and HC.
Limitations
Recruitment was in a single center, thus raising the possibility of population stratification.
Conclusions
The breadth of self-reproach and depressive cognitive style in evaluating events showed the same association with severity of depression in MDD and in post-COVID depressed patients, distributing along a gradient of severity, thus suggesting that individual features of negative thinking styles are shared in these conditions, and should be addressed as treatment targets in depressed COVID-19 survivors.
Keywords: COVID, Depression, Cognition, Neuropsychology, Bias, Distortion
1. Introduction
Negative thinking styles are a core feature of major depression, encompassing negative thinking, automatic thoughts, dysfunctional attributional style and dysfunctional attitudes, with irrational thought patterns in high level mental models used to interpret experience leading to self-deprecation, self-reproach, feelings of guilt, and a general negative outlook on the world and the self (Sheppard and Teasdale, 1996). Neuropsychological underpinnings of negative thinking styles include a mood-congruent processing bias toward negative stimuli, involving attention, memory, and processing speed. Opposite to healthy information processing, which is biased toward positive stimuli (Pool et al., 2016), during major depression there is a general facilitation of performance when responding to negative-tuned elements, with stimuli with a negative valence eliciting faster reaction times and causing robust brain activations in circuits related to emotional processing (Chamberlain and Sahakian, 2006; Elliott et al., 2000, Elliott et al., 2002; Leppänen, 2006; Matt et al., 1992).
According to the cognitive neuropsychological model of depression, distorted information processing, with a specific bias toward negative valence, critically contributes to sustain depressive psychopathology by fostering the experience of negative emotions and affects, and is a primary target for antidepressant treatment (Harmer et al., 2009; Pringle et al., 2011; Roiser et al., 2012). According to several authors, successful treatment of depression occurs when normal emotional information processing, and brain cortico-limbic neural responses implicated in the cognitive generation of affect, are restored (Benedetti et al., 2007; Benedetti et al., 2009; Davidson et al., 2003; Davidson et al., 2002; Vai et al., 2016; Vai et al., 2015).
Several medical conditions with brain and peripheral inflammation, and chronic pain, are associated with depression. A consistent literature described the same irrational thought patterns and cognitive errors both, in depressed patients with these conditions, and in major depressive disorder (Lefebvre, 1981; Maxwell et al., 1998; Smith et al., 1994; Smith et al., 1990; Szigethy et al., 2007), thus suggesting that negative thinking styles associates with clinical depression independent of factors triggering the major depressive episode. This is of high clinical relevance, because clinical investigations show that in medical and neurological conditions negative thinking styles worsen prognosis by increasing disability, when stage of disease is controlled (Benedetti et al., 2004; Chan et al., 2020; Clough, 1991; Mikocka-Walus et al., 2015; Smith et al., 1986). Cognitive vulnerability can moderate the effect of life stressors on depressive symptomatology, identifying a vulnerability factor (Beevers, 2005; Losiak et al., 2019), while a ‘normal’ bias toward positive emotional processing predicts stress resilience (Thoern et al., 2016).
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), involves a pneumonia with self-reinforcing ‘cytokine storms’, activation of innate immunity, and systemic inflammation (Fajgenbaum and June, 2020). Possibly due to the combined effects of the perturbation of the immune system, which can trigger major depression (Miller and Raison, 2016a), and of the stress of facing a potentially fatal disease, COVID-19 survivors often experience psychiatric sequelae including depression, anxiety, post-traumatic stress disorder, obsessive-compulsive symptoms, and insomnia, which persist after clinical recovery from the infection, and significantly contribute to the quality of life of survivors (Benedetti et al., 2020; De Lorenzo et al., 2020; Ma et al., 2020; Mazza et al., 2020, Mazza et al., 2021; Nie et al., 2020; Yuan et al., 2020). In the first three months after virus clearance, a large population study of medical records showed that mood disorders are the leading psychiatric diagnoses to require attention in COVID-19 survivors (Taquet et al., 2020), and our prospective cohort study showed that depressive psychopathology is persisting in survivors, is predicted by systemic inflammation, and is the main cause for administering psychopharmacological treatment in the follow-up (Benedetti et al., 2020; Mazza et al., 2020, Mazza et al., 2021).
Considering the above referenced literature, it can then be hypothesized that patients with post-COVID depression share the same mood-congruent negative thinking styles and cognitive vulnerability observed in major depressive disorder (MDD), which has relevant consequences in the clinics of mood disorders and of depression related to medical conditions. We tested this hypothesis by comparing cognitive measures in a sample of depressed COVID-19 survivors, compared with non-depressed COVID-19 survivors, healthy controls, and patients with MDD without COVID-19.
2. Methods
2.1. Participants and testing
We studied 729 participants, divided in four groups (Table 1 ): (1) 362 COVID-19 survivors, recruited in a naturalistic setting during an ongoing prospective cohort study at IRCCS San Raffaele Hospital in Milan, at mean ± SD 14.15 ± 7.89 weeks after clearance of the virus and hospital discharge, and divided in two groups according to the presence of depressive psychopathology; (2) 73 consecutively admitted inpatients affected by Major Depressive Disorder, current depressive episode without psychotic features, who served as positive controls for depressive psychopathology; and (3) 294 healthy participants recruited from the general population, who served as negative controls.
Table 1.
Demographic characteristics of the participants divided according to diagnosis, performance at the self-description task, and levels of significance of the observed differences (one-way ANOVA for the main group effect; and age-corrected GLM ANOVA for post-hoc comparisons between Post-COVID depressed patients and other groups). Values are means ± SD.
| Post Covid non-depressed (n = 281) – Group I |
Post-COVID depressed (n = 81) - Group II | Major depressive episode (n = 73) - Group III | Healthy controls (n = 294) - Group IV | F or χ2 | p | Post-hoc tests p-Values (Group II vs other groups) |
|||
|---|---|---|---|---|---|---|---|---|---|
| II VS I | II VS III | II VS IV | |||||||
| Sex (M/F) | 213/68 | 32/49 | 29/44 | 133/161 | 75.251 | <0.0001 | |||
| Age | 58.29 ± 11.17 | 58.17 ± 12.66 | 54.27 ± 12.96 | 40.46 ± 14.15 | 106.99 | <0.0001 | 0.9203 | 0.0235 | <0.0001 |
| Weeks after virus clearance | 13.62 ± 7.95 | 14.17 ± 7.85 | – | – | 0.30 | 0.583 | |||
| Frequency of attribution of positive elements | 87.31 ± 8.29 | 76.30 ± 11.62 | 51.59 ± 15.29 | 82.03 ± 9.19 | 256.93 | <0.0001 | <0.0001 | <0.0001 | 0.0011 |
| Frequency of attribution of negative elements | 12.69 ± 8.29 | 23.70 ± 11.62 | 48.41 ± 15.29 | 17.79 ± 9.13 | 258.65 | <0.0001 | <0.0001 | <0.0001 | 0.0011 |
| Latency of attribution of positive elements (msec) | 1640.7 ± 616.0 | 1844.4 ± 655.6 | 6220.2 ± 4454.6 | 1635.9 ± 504.0 | 200.36 | <0.0001 | 0.0070 | <0.0001 | 0.0939 |
| Latency of attribution of negative elements (msec) | 2279.9 ± 971.2 | 2306.7 ± 864.7 | 6025.0 ± 4166.4 | 2187.1 ± 830.7 | 125.75 | <0.0001 | 0.7778 | <0.0001 | 0.5488 |
| Ratio between latencies for positive and negative elements | 0.75 ± 0.17 | 0.82 ± 0.15 | 1.10 ± 0.60 | 0.78 ± 0.15 | 40.36 | <0.0001 | 0.0016 | <0.0001 | 0.0132 |
| ZSDS index score | 36.86 ± 6.08 | 58.11 ± 6.61 | 70.50 ± 10.23 | – | 793.12 | <0.0001 | <0.0001 | <0.0001 | – |
| Cognition Questionnaire | (n = 141) | (n = 47) | (n = 73) | – | |||||
| Total score | 10.27 ± 5.33 | 17.57 ± 9.61 | 26.15 ± 10.87 | – | 94.59 | <0.0001 | <0.0001 | 0.0001 | – |
| Emotional impact | 1.28 ± 1.56 | 2.81 ± 2.72 | 5.04 ± 3.11 | – | 64.11 | <0.0001 | <0.0001 | 0.0003 | – |
| Attribution of causality | 3.18 ± 1.88 | 4.43 ± 2.48 | 5.23 ± 2.44 | – | 22.84 | <0.0001 | 0.0004 | 0.1910 | – |
| Generalization across time | 1.45 ± 1.42 | 3.21 ± 2.31 | 5.11 ± 3.12 | – | 68.65 | <0.0001 | <0.0001 | 0.0020 | – |
| Generalization across situations | 3.11 ± 1.97 | 5.06 ± 2.68 | 6.34 ± 3.01 | – | 44.88 | <0.0001 | <0.0001 | 0.0604 | – |
| Perceived uncontrollability | 1.30 ± 1.72 | 2.06 ± 2.27 | 4.64 ± 2.87 | – | 56.21 | <0.0001 | 0.0196 | <0.0001 | – |
Diagnosis of COVID-19 was made after clinical and radiological findings suggestive of COVID-19 pneumonia at the admission to the Emergency Department, as confirmed by positive real-time reverse-transcriptase polymerase chain reaction from a nasopharyngeal and/or throat swab.
Severity of depressive psychopathology was self-rated by the patients on the Zung Self-Rating Depression Scale (ZSDS) (Zung, 1965), which proved a valid instrument to assess depression and need of antidepressant treatment in COVID-19 survivors (Mazza et al., 2021; Nie et al., 2020). The generally accepted standard cut-off scores was used to consider the presence of psychopathology (ZSDS index ≥50).
Neuropsychological bias toward emotional stimuli was tested in a self-description task, in which each subject was presented 50 positive and 50 negative, for a total of 100 morally tuned adjectives (e.g., brave/vile) in random order on a computer screen, and had to answer the question “Do these words apply to you?” by pressing keys. Recorded variables were frequencies of self-attributed positive or negative adjectives and reaction times. Positive adjectives rated as self-descriptive and negative adjectives rated as not self-descriptive were defined positive self-scheme elements; on the contrary, negative self-attributed and positive refused adjectives were defined as negative self-scheme elements. Previous studies showed that during major depression the frequency of elements with a negative moral valence is higher than in euthymic states at self-description. Moreover, the reaction time recorded in depressed patients is shorter for negative elements while it is longer for positive elements if compared to latencies exhibited by healthy subjects, with the ratio between the reaction times for the two providing a measure of the mood-congruent processing bias during a major depressive episode, sensitive to clinical improvement after treatment (Baving et al., 1997; Benedetti et al., 2005a, Benedetti et al., 2005b, Benedetti et al., 2007, Benedetti et al., 2016, Benedetti et al., 2008). The metrics derived from this task to be included in the data analyses were: [1] frequency of attribution of negative adjectives, and [2] the ratio between latencies of attribution for positive/negative adjectives.
In a subgroup of 188 COVID survivors and 73 hospitalized MD patients, negative thinking styles were also rated on the Cognition Questionnaire (CQ) (Fennell and Campbell, 1984), a measure of depressive cognitive style which assesses dimensions of negative thinking in relation to a number of hypothetical events. The questionnaire comprises five dimensions which are applied to the consequences of the hypothetical situations: emotional impact, attribution of causality, generalization across time, generalization across situations, perceived uncontrollability.
2.2. Statistical analyses
All the statistical analyses were performed with a commercially available software package (StatSoft Statistica 12, Tulsa, OK, USA) and following standard computational procedures (Dobson, 1990; Hill and Lewicki, 2006). All analyses were corrected for age and sex.
First, we performed statistical analyses to compare means and frequencies (ANOVA F-test, Pearson χ2 test) exploring the effect of group on differences in response latencies, frequencies of negative elements at self-description, depressive cognitive style, severity of depressive symptoms (See Table 1). Post-hoc Least Significance Difference (LSD) tests were also computed as appropriate. To further investigate the effect of diagnostic group on the aforementioned constructs a Generalized Linear Model (GLZM) analysis was also performed, while controlling for age and sex. Again, post-hoc Kruskal-Wallis tests were conducted to detect any between groups difference.
Second, in order to explore the relationship between cognitive vulnerability and depressive psychopathology, three separate GLMZ homogeneity of slope regression analyses were modelled: i) ratio between latencies to respond to positive/negative elements, and group, were entered as predictors of number of self-attributed negative elements; ii) ratio between latencies and group were entered as predictors of severity of depressive symptoms; iii) number of negative self-scheme elements were entered as predictors of depressive symptoms. All the analysis considered age and sex as nuisance covariates. In case of significant interaction between predictors, additional GLMZ separate slope analysis was conducted.
Third, considering the results of the GLZM analyses, we tested a mediation/moderation model of the predictive effect of the neuropsychological metrics (ratio between latencies to respond to positive/negative elements) on severity of depression, with number of negative self-scheme elements as mediator, and group (COVID survivors vs MD patients) as moderator, by using the nonparametric resampling procedure implemented in Process v.4.0, as integrated in IBM Statistical Package for Social Science (SPSS) v23.0 (https://www.processmacro.org/index.html) (Hayes, 2017). Briefly, the model tested the conditional indirect effect of the predictor on the dependent variable (a x b), after testing (i) for the interaction effect of the moderator with the predictor, and (ii) for the main effects of the predictor on the mediator (a), and of the mediator on the dependent variable (b) in the two diagnostic groups (see Hayes, 2017; Model 58, p.683). 5000 bootstrap resamples were used to generate 95% confidence percentile intervals for each analysis.
Fourth, in the subgroup in which depressive cognitive style was assessed with CQ, three separates GLMZ homogeneity of slope regression analyses were run to examine the association of depressive cognitive style as assessed through CQ with depressive symptoms as assessed through ZSDS, and the two measures returned from the self-description task (i.e., frequency of negative elements, and ratio between latencies), always considering age and sex as nuisance covariates. We specified the following three different models: i) CQ score and group were entered as predictors of depressive severity; ii) frequency of negative elements and group were entered as predictors of CQ score; iii) ratio between latencies in self-attribution and group were entered as predictors of CQ score.
For all the GLZM analyses of homogeneity of slopes or separate-slopes regression as appropriate, with used an identity link function (McCullagh and Nelder, 1989). Parameter estimates were obtained with iterative re-weighted least squares maximum likelihood procedures. The significance of the effects was calculated with the likelihood ratio (LR) statistic, which provides the most asymptotically efficient test known, by performing sequential tests for the effects in the model of the factors on the dependent variable, at each step adding an additional effect into the model contributing to incremental Chi-square statistic, thus providing a test of the increment in the log-likelihood attributable to each current estimated effect; or the Wald W2 test as appropriate (Agresti, 1996; Dobson, 1990). Homogeneity of slopes was tested for the interaction of continuous and categorical variables, and separate-slopes regression was performed to test if the relationship between predictors and outcomes was the same in diagnostic groups. The quality of the statistical models was checked using the entropy maximization principle of the Akaike information criterion (AIC) (Akaike, 1974).
3. Results
First, clinical and demographic characteristics of the sample are resumed in Table 1. 81/362 (22.4%) COVID survivors self-rated their depressive symptoms above the clinical threshold (ZSDS = 50), with a higher prevalence of depression among females (41.9% vs 13.1%, χ2 = 37.86, p < 0.0001) which confirmed previous epidemiological observations on gender ratio in post-COVID depression (Mazza et al., 2020, Mazza et al., 2021). Both post-COVID depressed patients and MDD shared a 1.5:1 F:M gender ratio. 10/81 COVID patients with ZSDS >50 had a previous positive history of MDD, and did not significantly differ from the other depressed COVID survivors on any test measure (Table 1).
Self-rating depression above the clinical threshold (ZSDS >50) significantly associated with more negative elements at self-description, and faster relative reaction times for negative elements, with post-COVID depressed patients showing intermediate scores between MDD patients, and non-depressed post-COVID participants and HC (Table 1). Group effects on frequency of attribution of negative elements were tested in a GLZM analysis corrected for age and sex showed significant (Whole model AIC for better subset: LR χ2 = 538.58, p < 0.0001; Group effect: χ2 = 505.77, p < 0.0001). Group also significantly influenced the ratio between latencies of attribution of positive/negative elements (Whole model AIC for better subset: LR χ2 = 132.03, p < 0.0001; χ2 = 108.54, p < 0.0001). Kruskal-Wallis post-hoc multiple comparisons testing showed that depressed post-COVID patients self-attributed significantly more negative elements than post-COVID non-depressed (z′ = 8.28, p < 0.0001) and HC (z′ = 3.72, p = 0.0011), and less than MDD patients (z′ = 5.93, p < 0.0001); and showed a ratio between latencies of attribution higher than post-COVID non-depressed (z′ = 3.25, p = 0.0069), and lower than MDD (z′ = 4.08, p = 0.0003). When adding severity of depression to the model (ZSDS scores), group effects remained highly significant, and ZSDS significantly influenced the frequency of attribution of negative elements (χ2 = 63.15, p < 0.0001).
Second, the difference in reaction times to self-attribute elements with positive and negative moral valence (ratio between latencies to self-attribute positive/negative elements) significantly predicted self-description in all groups (Fig. 1 , top): higher positive/negative ratio, higher number of negative self-scheme elements. A GLZM separate-slopes analysis showed that this relationship was significant in all groups (COVID depressed: Wald W2 = 19.81; COVID non depressed: W2 = 15.48; MD: W2 = 13.65; HC: W2 = 33.54; all p < 0.001), but with a highly significant group interaction (LR χ2 = 486.92, p < 0.0001). Decomposition of single effects showed that the relationship between latencies ratio and self-description did not follow parallel slopes between depressed and non-depressed participants, being steeper in depressed COVID survivors (versus non depressed COVID W2 = 53.92; versus HC W2 = 69.13; all p < 0.0001) and in MD patients (versus non depressed COVID W2 = 49.20; versus HC W2 = 57.94; all p < 0.0001). The slopes were parallel in depressed COVID survivors and in MD patients, but MDD patients showed worse self-reproach (main effect of group: W2 = 10.21; p = 0.0014), and a stronger effect of processing bias on self-description (worse negative moral self-description at similar levels of processing bias, group interaction W2 = 96.63; p < 0.0001).
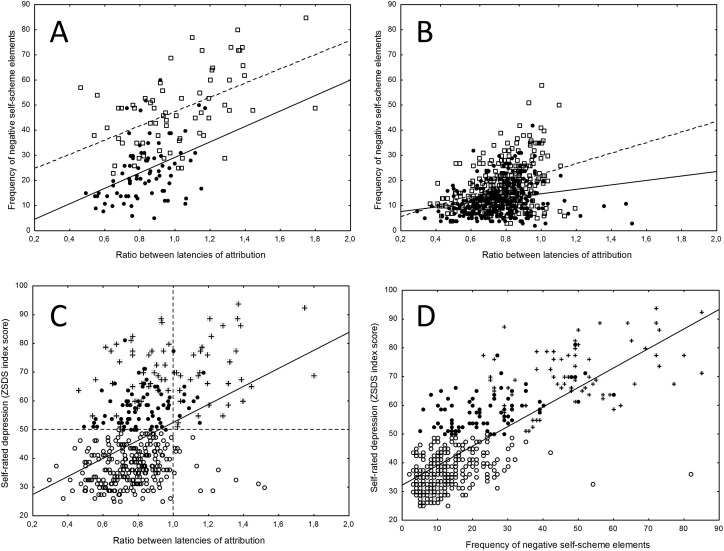
Fig. 1.
Effects of the mood-congruent bias in latency to self-attribute positive and negative morally tuned adjectives (ratio between latencies to attribute positive/negative), and frequency of self-attribution of morally negative self-descriptive elements, on severity of depression and negative thinking styles. A: effect of ratio between latencies on frequency of attribution of negative self-scheme elements in patients with depressive ratings above the clinical threshold (ZSDS = 50). Black dots = COVID survivors (continous line fitting); White dots = hospitalized MD patients (dotted line fitting). B: effect of ratio between latencies on frequency of attribution of negative self-scheme elements in COVID survivors without depression (black dots, continous line fitting) and in HC (white dots, dotted line fitting). C: effect of ratio between latencies on ZSDS scores, with linear least-squares fitting and thresholds for the presence of clinical depression and of information processing bias (dotted lines). White dots: COVID survivors without depression; Black dots: depressed COVID survivors; Stars: hospitalized MD patients. D: effect of frequency of attribution of negative self-scheme elements on ZSDS score.
Moreover, the ratio between latencies to self-attribute positive/negative elements strongly predicted the severity of self-rated depressive psychopathology (ZSDS scores) (LR χ2 = 40.99, p < 0.0001) (Fig. 1, bottom). Interestingly, the equation for linear fitting was y = 21.2 + 31.3x (x = ratio between latencies, y = ZSDS score), thus predicting ZSDS values very close to the 50 cutoff when latency to respond was not biased toward positive or negative moral self-scheme elements (i.e., when ratio = 1).
Stratification of diagnostic groups showed that the ratio between latencies in COVID survivors and MDD patients distributed along the same gradient, and testing homogeneity of slopes yielded not significant group interactions for the effect of the processing bias on depression severity (Wald W2 = 0.52, p = 0.473). In turn, also the frequency of morally negative elements at self-description strongly predicted ZSDS scores (LR χ2 = 127.89, p < 0.0001), and again, testing homogeneity of slopes yielded not significant group interactions between COVID survivors and hospitalized MDD patients in the relationship between self-loathing and ZSDS scores (Wald W2 = 2.11, p = 0.146).
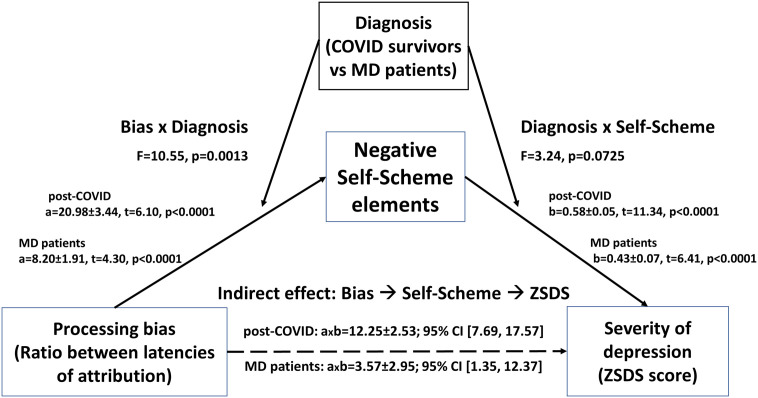
Third, given the significant relationships between ratio between latencies, negative self-description, and depressive symptomatology, we tested a mediation/moderation model of the effect of ratio between latencies on severity of depression, with frequency of attribution of negative self-scheme elements as mediator, and group (COVID survivors vs MDD patients) as moderator of the relationship between ratio between latencies and depression (Fig. 2 ).
Fig. 2.
Mediation/moderation model of the effect of cognitive vulnerability (ratio between latencies to attribute positive/negative elements; and frequency of attribution of negative self-scheme elements) on severity of depression. a, b, axb: extimated coefficients for the effect of factors on outcomes, and their interaction, ±standard errors.
The model showed a high performance (for the effect of ratio between latencies on negative self-scheme, R2 = 0.589, F = 119.69, d.f. 5417, p < 0.0001; for the combined effect of the ratio between latencies and the negative self-scheme on depressive symptomatology, R2 = 0.634, F = 119.84, d.f. 6416, p < 0.0001). Results confirmed significant effects of factors on outcomes in both groups, and the significant moderation effect of diagnosis on the relationship between ratio between latencies and negative self-scheme elements, but not on the relationship between negative self-scheme elements and depressive symptomatology. Indirect effects (ratio between latencies ➔ negative self-scheme elements ➔ severity of depression) were significant in both groups, and not different among them: when estimating bootstrapped 95% confidence intervals for coefficients, the mediation was significant in both groups, while the moderated mediation was not (index = −8.69 ± 3.98, 95% CI = −14.32, 1.57), meaning that the difference between conditional indirect effects in the two groups was not significantly different from zero.
Finally, depressive cognitive style in evaluation of hypothetical events (Cognition Questionnaire scores) in depressed post-COVID patients showed intermediate levels of severity in all dimensions between non-depressed post-COVID patients, and MDD (Table 1, bottom; post-hoc Fisher's least significance test: p < 0.05 at all comparisons). The CQ total score significantly influenced the severity of depressive symptomatology as rated on the ZSDS (χ2 = 84.60, p < 0.0001), with COVID survivors and MDD patients distributing along the same gradient of progressive severity, leading to a significant group effect (worse scores in MDD patients, χ2 = 172.76, p < 0.0001), and with no significant group x CQ scores interaction (χ2 = 1.19, p = 0.276) (Table 1, Fig. 3 ).
Fig. 3.
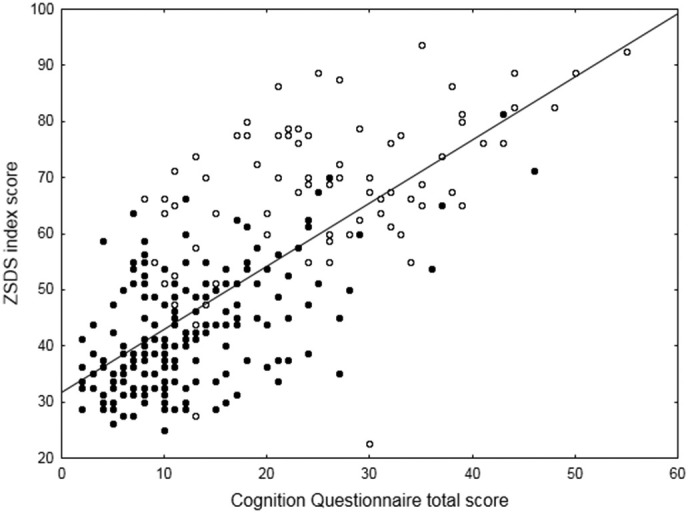
Effect of depressive cognitive style in evaluation of hypothetical events (Cognition Questionnaire score) on the severity of depressive symptomatology as self-rated on the ZSDS. Black dots: COVID patients. White dots: MDD patients.
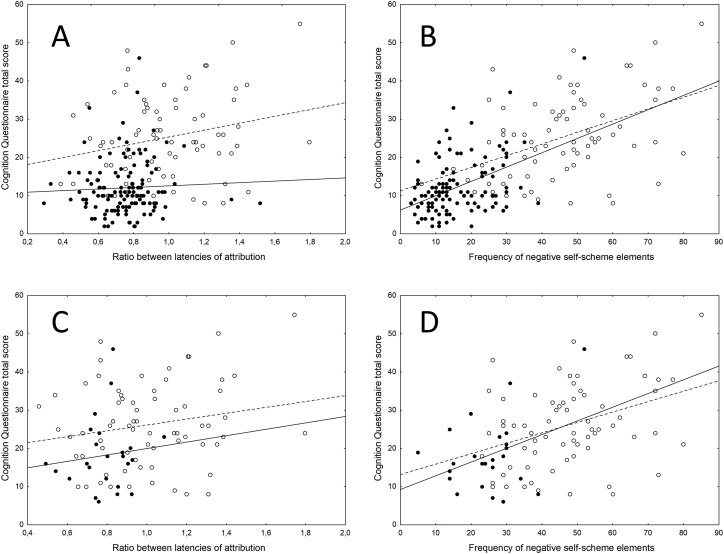
The CQ score was predicted both, by the ratio between latencies to self-attribute positive/negative elements (χ2 = 3.91, p = 0.0479), and by the frequency of attribution of negative self-scheme elements (χ2 = 42.96, p < 0.0001), with a significant main effect of group (post-COVID vs MD: χ2 = 91.37, p < 0.0001) when considering MDD patients and both, depressed and non-depressed COVID survivors together. Inspection of data (Fig. 4 ) shows a stronger dependency of CQ scores from ratio between latencies of attribution in MDD patients than in COVID survivors, when considering all COVID patients (Fig. 4, top); while, when considering depressed COVID survivors only (Fig. 4, bottom), the relationship was similar in the two groups. The association of negative self-description with CQ scores followed a similar relationship in the two groups.
Fig. 4.
Effect of cognitive vulnerability (ratio between latencies to attribute positive/negative elements; and frequency of attribution of negative self-scheme elements) on Cognition Questionnaire scores when considering MD patients and all COVID patients (Top); and when considering MD patients and depressed COVID survivors only (bottom). Black dots, continous line: COVID patients. White dots, dotted line fitting: MD patients.
4. Discussion
This is the first study investigating the mood-congruent negative thinking styles in moral self-description, latency to respond to positive or negative morally tuned adjectives, and depressive cognitive style in causal attribution and interpretation of hypothetical events, in patients with post-COVID depression.
The main finding is that, when compared with patients with an ongoing major depressive episode, depressed COVID survivors show a similar pattern of relationships between mood-congruent negative thinking styles and the severity of the depressive syndrome, distributing along a gradient of severity, with both groups showing a significantly different performance in respect to healthy controls and non-depressed COVID patients: thus suggesting that in both depressive conditions it is the presence of depressive psychopathology that is associated with a preponderance of negative thinking, proportional to its severity, and irrespective of its post-COVID origin.
In particular, in respect to HC and to non-depressed COVID patients, post-COVID depressed patients used more morally negative adjectives to describe themselves, and showed lower latencies to assess their self-relatedness; moreover, they showed worse depressive cognitive style and negative thinking when evaluating a number of hypothetical events. In respect to hospitalized patients with MDD, they showed a lower severity of self-rated depression, paralleling less severe mood-congruent negative thinking styles. The main difference between MDD and post-COVID depressed patients was in the relationship between the neuropsychological mood-congruent processing bias toward negative stimuli, and severity of the depressive syndrome and of other dimensions of cognitive vulnerability, which was stronger in patients with MDD. However, the breadth of moral self-reproach and the severity of negative thinking styles in evaluating events showed the same association with severity of depression in the two groups.
This observation has clinical implications. A consistent literature showed that in MDD self-esteem, negative thinking, and dysfunctional attitudes predict duration of the depressive episodes (Williams et al., 1990), recovery (Bothwell and Scott, 1997), hopelessness (Cannon et al., 1999) and suicidality (Whisman et al., 1995); they influence decision making thus impacting quality of life (Mukherjee et al., 2020); and they correlate with subsequent depression (Kernis et al., 1991), change very slowly (Fennell and Campbell, 1984), may persist undetected after recovery in formerly depressed patients (Hedlund and Rude, 1995), and can be targeted by specific therapeutic interventions (Segal et al., 1999). If post-COVID depression shares these psychopathological features with MDD, as it is observed when depression is triggered by other medical conditions (see Introduction), they are likely to influence its outcome as well, and should then be considered as a target for treatment.
COVID-19 induces a ‘cytokine storm’ involving massive release of pro-inflammatory cytokines (Fajgenbaum and June, 2020). An extensive literature associated severity and outcome of MDD with an abnormal setpoint of pro-inflammatory immune/inflammatory setpoint, leading to persistent low-grade inflammation. The hyperinflammatory state and the subsequent cytokine dysregulation may affect interaction pathways between the immune system and psychopathological mechanisms, also including a persistent perturbation of brain monoaminergic neurotransmission (Arteaga-Henríquez et al., 2019; Dantzer, 2018; Hodes et al., 2015; Miller and Raison, 2016b). The raised pro-inflammatory cytokines typically associated with the prognosis of MDD such as Interleukin(IL)-1β and IL-6 (Benedetti et al., 2021; Poletti et al., 2020), have been recently linked also to post-COVID depression and its prevention (Benedetti et al., 2020). Alterations in blood cell counts associated with inflammation (e.g., neutrophils, lymphocytes, platelets) are also associated with MDD (Mazza et al., 2018), and predict depressive psychopathology in COVID-19 survivors (Mazza et al., 2020), with time-dependent variations of these indexes predicting the improvement or worsening of depressive symptoms over time (Mazza et al., 2021). It can be surmised that shared psychopathological features in MDD and post-COVID depression could be underpinned by shared inflammatory mechanisms in the two conditions. The interest for further research, comparing inflammatory biomarkers in MDD and post-COVID depression and possibly associating them with psychopathological features, is warranted.
The present results must be viewed in light of some limitations. The limited health care resources and patient's compliance related to the clinical setting forced us to choose an unstructured interview format instead of a structured clinical interview for psychopathological assessment. Moreover, we were not able to assess all dimensions of cognitive vulnerability in all patients, but only in a subsample. Recruitment was in a single center, thus raising the possibility of population stratification and therefore limiting the possibility to generalize our conclusions. Lastly, the cross-sectional design of the current study makes it challenging to infer causality between the affective/cognitive aspects of post COVID depression. These limitations, however, do not bias the main finding that post-COVID depression shares cognitive vulnerability and negative thinking styles in self-reproach and evaluation of events, similar to those observed in MDD, thus suggesting that these individual features should be addressed as treatment targets in personalizing interventions. In the face to reshape psychotherapeutic practice in the post-COVID-19 era, we highly encourage clinicians to tailor cognitive-therapy protocols which may potentially reverse specific thinking errors and hence promoting a full-recovery of COVID-19 survivors.
CRediT authorship contribution statement
FB, MGM, and MP designed the study, and carried it out with contributions from all authors. FB analyzed the results and wrote the first draft, which was critically reviewed and approved by all authors.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgements
Funding
MGM salary: Italian Ministry of University, XXXV PhD cycle. MP salary: Italian Ministry of University, XXXVII PhD cycle, FSE REACT-EU 2021 PON projects, Action IV.5.
The COVID-19 BioB Outpatient Clinic Study group also includes: Bollettini Irene, Bosio Sara, Bravi Beatrice, Bussolari Cecilio, Calvisi Stefania, Canti Valentina, Caselani Elisa, Castellani Jacopo, Cilla Marta, Cinel Elena, Colombo Federica, Damanti Sarah, Di Pasquasio Camilla, Ferrante Marica, Fiore Paola, Fumagalli Anna, Magnaghi Cristiano, Martinenghi Sabina, Mazza Elena Beatrice, Melloni Elisa Maria Teresa, Merolla Aurora, Pomaranzi Chiara, Santini Chiara, Vai Benedetta, Vitali Giordano.
References
- Agresti A. Wiley; New York: 1996. An Introduction to Categorical Data Analysis. [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974;19:716–723. [Google Scholar]
- Arteaga-Henríquez G., Simon M.S., Burger B., Weidinger E., Wijkhuijs A., Arolt V., Birkenhager T.K., Musil R., Müller N., Drexhage H.A. Low-grade inflammation as a predictor of antidepressant and anti-inflammatory therapy response in MDD patients: a systematic review of the literature in combination with an analysis of experimental data collected in the EU-Moodinflame Consortium. Front. Psychiatry. 2019;10 doi: 10.3389/fpsyt.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baving L., Maes H., Bohus M., Lis S., Krieger S., Olbrich H., Berger M. Can negative self-schemes in depressives be altered through sleep deprivation? J. Affect. Disord. 1997;42:93–101. doi: 10.1016/s0165-0327(96)01401-2. [DOI] [PubMed] [Google Scholar]
- Beevers C.G. Cognitive vulnerability to depression: a dual process model. Clin. Psychol. Rev. 2005;25:975–1002. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Campori E., Colombo C., Smeraldi E. Fluvoxamine treatment of major depression associated with multiple sclerosis. J. Neuropsychiatry Clin. Neurosci. 2004;16:364–366. doi: 10.1176/jnp.16.3.364. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Barbini B., Cigala Fulgosi M., Pontiggia A., Colombo C., Smeraldi E. Cognitive assessment of depression: a new test for mood disorders. Clin. Psychiatry. 2005;2:149–165. [Google Scholar]
- Benedetti F., Barbini B., Florita M., Cigala Fulgosi M., Campori E., Colombo C., Smeraldi E. Rapid improvement in information processing after sleep deprivation and sleep phase-advance in bipolar depression. Clin. Psychiatry. 2005;2:180–182. [Google Scholar]
- Benedetti F., Bernasconi A., Blasi V., Cadioli M., Colombo C., Falini A., Lorenzi C., Radaelli D., Scotti G., Smeraldi E. Neural and genetic correlates of antidepressant response to sleep deprivation - a functional magnetic resonance imaging study of moral valence decision, in bipolar depression. Arch. Gen. Psychiatry. 2007;64:179–187. doi: 10.1001/archpsyc.64.2.179. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Radaelli D., Bernasconi A., Dallaspezia S., Falini A., Scotti G., Lorenzi C., Colombo C., Smeraldi E. Clock genes beyond the clock: CLOCK genotype biases neural correlates of moral valence decision in depressed patients. Genes Brain Behav. 2008;7:20–25. doi: 10.1111/j.1601-183X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Radaelli D., Bernasconi A., Dallaspezia S., Colombo C., Smeraldi E. Changes in medial prefrontal cortex neural responses parallel successful antidepressant combination of venlafaxine and light therapy. Arch. Ital. Biol. 2009;147:83–94. [PubMed] [Google Scholar]
- Benedetti F., Poletti S., Hoogenboezem T.A., Locatelli C., Ambree O., de Wit H., Wijkhuijs A.J., Mazza E., Bulgarelli C., Vai B., Colombo C., Smeraldi E., Arolt V., Drexhage H.A. Stem cell factor (SCF) is a putative biomarker of antidepressant response. J. NeuroImmune Pharmacol. 2016;11:248–258. doi: 10.1007/s11481-016-9672-y. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Mazza M., Cavalli G., Ciceri F., Dagna L., Rovere-Querini P. Can cytokine blocking prevent depression in COVID-19 survivors? J. NeuroImmune Pharmacol. 2020:1–3. doi: 10.1007/s11481-020-09966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Poletti S., Vai B., Mazza M.G., Lorenzi C., Brioschi S., Aggio V., Branchi I., Colombo C., Furlan R. Higher baseline interleukin-1β and TNF-α hamper antidepressant response in major depressive disorder. Eur. Neuropsychopharmacol. 2021;42:35–44. doi: 10.1016/j.euroneuro.2020.11.009. [DOI] [PubMed] [Google Scholar]
- Bothwell R., Scott J. The influence of cognitive variables on recovery in depressed inpatients. J. Affect. Disord. 1997;43:207–212. doi: 10.1016/s0165-0327(97)01431-6. [DOI] [PubMed] [Google Scholar]
- Cannon B., Mulroy R., Otto M.W., Rosenbaum J.F., Fava M., Nierenberg A.A. Dysfunctional attitudes and poor problem solving skills predict hopelessness in major depression. J. Affect. Disord. 1999;55:45–49. doi: 10.1016/s0165-0327(98)00123-2. [DOI] [PubMed] [Google Scholar]
- Chamberlain S.R., Sahakian B.J. The neuropsychology of mood disorders. Curr. Psychiatry Rep. 2006;8:458–463. doi: 10.1007/s11920-006-0051-x. [DOI] [PubMed] [Google Scholar]
- Chan C.K., Tian F., Pimentel Maldonado D., Mowry E.M., Fitzgerald K.C. Depression in multiple sclerosis across the adult lifespan. Mult. Scler. J. 2020;1352458520979304 doi: 10.1177/1352458520979304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough D.H. The effects of cognitive distortion and depression on disability in rheumatoid arthritis. Res. Nurs. Health. 1991;14:439–446. doi: 10.1002/nur.4770140608. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 2018;98:477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Pizzagalli D., Nitschke J.B., Putnam K. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Irwin W., Anderle M.J., Kalin N.H. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am. J. Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., Brioni E., Giacalone G., Canti V., Sofia V. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.J. Chapman & Hall; New York: 1990. An introduction to generalized linear models. [Google Scholar]
- Elliott R., Rubinsztein J.S., Sahakian B.J., Dolan R.J. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Elliott R., Rubinsztein J.S., Sahakian B.J., Dolan R.J. The neural basis of mood-congruent processing biases in depression. Arch. Gen. Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell M.J., Campbell E.A. The cognitions questionnaire: specific thinking errors in depression. Br. J. Clin. Psychol. 1984;23(Pt 2):81–92. doi: 10.1111/j.2044-8260.1984.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Harmer C.J., Goodwin G.M., Cowen P.J. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br. J. Psychiatry. 2009;195:102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Guilford Press; 2017. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. [Google Scholar]
- Hedlund S., Rude S.S. Evidence of latent depressive schemas in formerly depressed individuals. J. Abnorm. Psychol. 1995;104:517. doi: 10.1037//0021-843x.104.3.517. [DOI] [PubMed] [Google Scholar]
- Hill T., Lewicki P. General Linear Models. StatSoft; Tulsa (OK): 2006. Statistics: methods and applications. A comprehensive reference for science, industry, and data mining; pp. 245–276. Chapter 18. [Google Scholar]
- Hodes G.E., Kana V., Menard C., Merad M., Russo S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015;18:1386. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernis M.H., Grannemann B.D., Mathis L.C. Stability of self-esteem as a moderator of the relation between level of self-esteem and depression. J. Pers. Soc. Psychol. 1991;61:80. doi: 10.1037//0022-3514.61.1.80. [DOI] [PubMed] [Google Scholar]
- Lefebvre M.F. Cognitive distortion and cognitive errors in depressed psychiatric and low back pain patients. J. Consult. Clin. Psychol. 1981;49:517. doi: 10.1037//0022-006x.49.4.517. [DOI] [PubMed] [Google Scholar]
- Leppänen J.M. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr. Opin. Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Losiak W., Blaut A., Kłosowska J., Losiak-Pilch J. Stressful life events, cognitive biases, and symptoms of depression in young adults. Front. Psychol. 2019;10:2165. doi: 10.3389/fpsyg.2019.02165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.F., Li W., Deng H.B., Wang L., Wang Y., Wang P.H., Bo H.X., Cao J., Zhu L.Y., Yang Y., Cheung T., Ng C.H., Wu X., Xiang Y.T. Prevalence of depression and its association with quality of life in clinically stable patients with COVID-19. J. Affect. Disord. 2020;275:145–148. doi: 10.1016/j.jad.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt G.E., Vázquez C., Campbell W.K. Mood-congruent recall of affectively toned stimuli: a meta-analytic review. Clin. Psychol. Rev. 1992;12:227–255. [Google Scholar]
- Maxwell T.D., Gatchel R.J., Mayer T.G. Cognitive predictors of depression in chronic low back pain: toward an inclusive model. J. Behav. Med. 1998;21:131–143. doi: 10.1023/a:1018723823523. [DOI] [PubMed] [Google Scholar]
- Mazza M.G., Lucchi S., Tringali A.G.M., Rossetti A., Botti E.R., Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: a meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;84:229–236. doi: 10.1016/j.pnpbp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F., Group C.-B.O.C.S. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain. Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P., Nelder J.A. 2nd ed. Chapman & Hall; New York: 1989. Generalized Linear Models. [Google Scholar]
- Mikocka-Walus A., Bampton P., Hetzel D., Hughes P., Esterman A., Andrews J.M. Cognitive-behavioural therapy has no effect on disease activity but improves quality of life in subgroups of patients with inflammatory bowel disease: a pilot randomised controlled trial. BMC Gastroenterol. 2015;15:54. doi: 10.1186/s12876-015-0278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D., Lee S., Kazinka R., Satterthwaite T.D., Kable J.W. Multiple facets of value-based decision making in major depressive disorder. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-60230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X.-D., Wang Q., Wang M.-N., Zhao S., Liu L., Zhu Y.-L., Chen H. Anxiety and depression and its correlates in patients with coronavirus disease 2019 in Wuhan. Int. J. Psychiatry Clin. Pract. 2020:1–6. doi: 10.1080/13651501.2020.1791345. [DOI] [PubMed] [Google Scholar]
- Poletti S., Vai B., Mazza M.G., Zanardi R., Lorenzi C., Calesella F., Cazzetta S., Branchi I., Colombo C., Furlan R. A peripheral inflammatory signature discriminates bipolar from unipolar depression: a machine learning approach. Prog. Neuropsychopharmacol. Biol. 2020;Psychiatry doi: 10.1016/j.pnpbp.2020.110136. [DOI] [PubMed] [Google Scholar]
- Pool E., Brosch T., Delplanque S., Sander D. Attentional bias for positive emotional stimuli: a meta-analytic investigation. Psychol. Bull. 2016;142:79. doi: 10.1037/bul0000026. [DOI] [PubMed] [Google Scholar]
- Pringle A., Browning M., Cowen P., Harmer C. A cognitive neuropsychological model of antidepressant drug action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1586–1592. doi: 10.1016/j.pnpbp.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Roiser J.P., Elliott R., Sahakian B.J. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37:117–136. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal Z.V., Gemar M., Williams S. Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. J. Abnorm. Psychol. 1999;108:3. doi: 10.1037//0021-843x.108.1.3. [DOI] [PubMed] [Google Scholar]
- Sheppard L.C., Teasdale J.D. Depressive thinking: changes in schematic mental models of self and world. Psychol. Med. 1996;26:1043–1051. doi: 10.1017/s0033291700035364. [DOI] [PubMed] [Google Scholar]
- Smith T.W., Follick M.J., Ahern D.K., Adams A. Cognitive distortion and disability in chronic low back pain. Cogn. Ther. Res. 1986;10:201–210. [Google Scholar]
- Smith T.W., Peck J.R., Ward J.R. Helplessness and depression in rheumatoid arthritis. Health Psychol. 1990;9:377. doi: 10.1037//0278-6133.9.4.377. [DOI] [PubMed] [Google Scholar]
- Smith T.W., O'Keeffe J.L., Christensen A.J. Cognitive distortion and depression in chronic pain: association with diagnosed disorders. J. Consult. Clin. Psychol. 1994;62:195. doi: 10.1037//0022-006x.62.1.195. [DOI] [PubMed] [Google Scholar]
- Szigethy E., Kenney E., Carpenter J., Hardy D.M., Fairclough D., Bousvaros A., Keljo D., Weisz J., Beardslee W.R., Noll R. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:1290–1298. doi: 10.1097/chi.0b013e3180f6341f. [DOI] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2020;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoern H.A., Grueschow M., Ehlert U., Ruff C.C., Kleim B. Attentional bias towards positive emotion predicts stress resilience. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0148368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vai B., Poletti S., Radaelli D., Dallaspezia S., Bulgarelli C., Locatelli C., Bollettini I., Falini A., Colombo C., Smeraldi E., Benedetti F. Successful antidepressant chronotherapeutics enhance fronto-limbic neural responses and connectivity in bipolar depression. Psychiatry Res. 2015;233:243–253. doi: 10.1016/j.pscychresns.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Vai B., Bulgarelli C., Godlewska B.R., Cowen P.J., Benedetti F., Harmer C.J. Fronto-limbic effective connectivity as possible predictor of antidepressant response to SSRI administration. Eur. Neuropsychopharmacol. 2016;26:2000–2010. doi: 10.1016/j.euroneuro.2016.09.640. [DOI] [PubMed] [Google Scholar]
- Whisman M.A., Miller I.W., Norman W.H., Keitner G.I. Hopelessness depression in depressed inpatients: symptomatology, patient characteristics, and outcome. Cognit. Ther. Res. 1995;19:377–398. [Google Scholar]
- Williams J., Healy D., Teasdale J., White W., Paykel E. Dysfunctional attitudes and vulnerability to persistent depression. Psychol. Med. 1990;20:375–381. doi: 10.1017/s0033291700017694. [DOI] [PubMed] [Google Scholar]
- Yuan B., Li W., Liu H., Cai X., Song S., Zhao J., Hu X., Li Z., Chen Y., Zhang K. Correlation between immune response and self-reported depression during convalescence from COVID-19. Brain, Behavior, and Immunity. 2020;88:39–43. doi: 10.1016/j.bbi.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung W.W. A self-rating depression scale. Arch. Gen. Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]