Abstract
The disproportionate evolutionary expansion of the human cerebral cortex with reinforcement of cholinergic innervations warranted a major rise in the functional and metabolic load of the conserved basal forebrain (BF) cholinergic system. Given that acetylcholine (ACh) regulates properties of the microtubule-associated protein (MAP) tau and promotes non-amyloidogenic processing of amyloid precursor protein (APP), growing neocortex predicts higher demands for ACh, while the emerging role of BF cholinergic projections in Aβ clearance infers greater exposure of source neurons and their innervation fields to amyloid pathology. The higher exposure of evolutionary most recent cortical areas to the amyloid pathology of Alzheimer’s disease (AD) with synaptic impairments and atrophy, therefore, might involve attenuated homeostatic effects of BF cholinergic projections, in addition to fall-outs of inherent processes of expanding association areas. This unifying model, thus, views amyloid pathology and loss of cholinergic cells as a quid pro quo of the allometric evolution of the human brain, which in combination with increase in life expectancy overwhelm the fine homeostatic balance and trigger the disease process.
Keywords: Brain evolution, Cholinergic neurons, p75 NTR, Amyloid deposition, Cortical expansion, Default mode networks, Alzheimer’s disease
1. Introduction
In 1884 Croonian Lectures on the Evolution and Dissolution of the Nervous System, Hughlings Jackson asserted that ‘on account of the great preponderance of the highest centers in man, he differs so greatly from lower animals.. we can have a combination.. (of diseases).. never actually experienced’ [1], implying that some neurological and psychiatric conditions can be viewed from an evolutionary perspective as human-specific. Amongst the largely or exclusively human-specific brain diseases, frontal dementia, schizophrenia, and Alzheimer’s disease (AD) are most frequently discussed [2–4]. The bona fide AD-like neuro-behavioural symptoms and associated pathological changes are very rare in other animals, including in our closest evolutionary relative primates, whereas in humans they are common in old age. The sporadic form of the disease typically sets on after 65 years of age and its prevalence doubles every five years. In developed countries in over 85-year--old populations, the occurrence of AD ranges from 20 to 40 % [5,6].
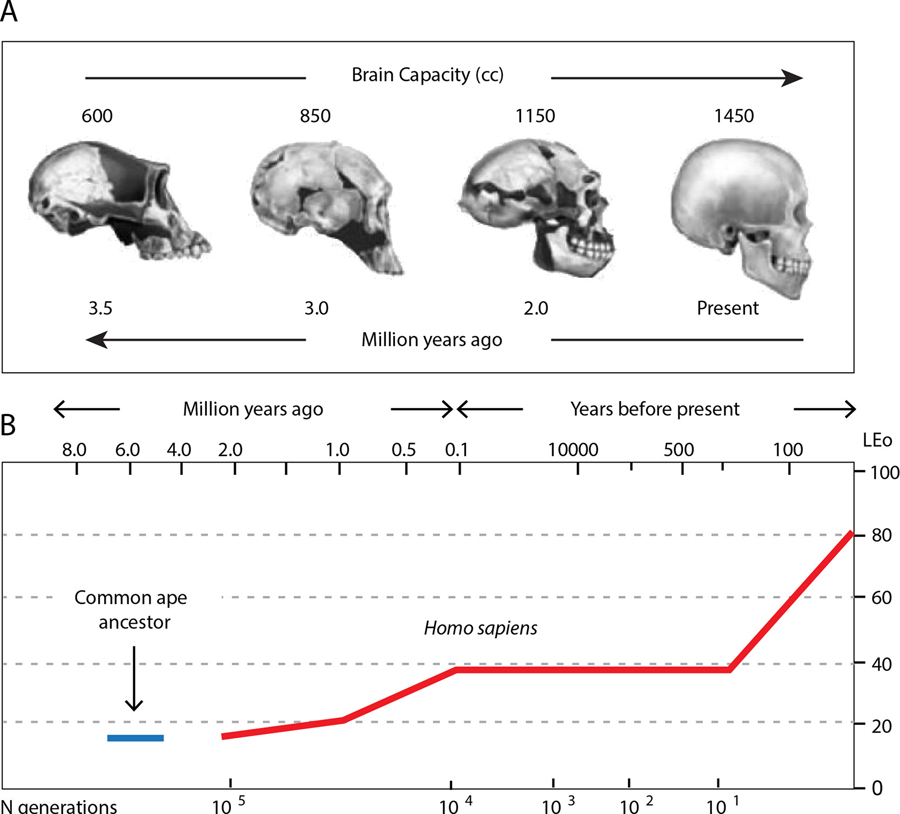
In support of H. Jackson’s thesis, many recent studies have viewed the disproportionate enlargement of the evolutionary most progressive and dynamic cortical areas as a key for the prevalence of AD in humans [7–9]. Clinically, the disease manifests via an irreversible decline in higher mental faculties, including executive and cognitive functions, working and declarative memory, language, computation skills, judgment and social intelligence. While present in various animals, these functions have advanced dramatically in humans in parallel with the expansion of association cortical networks. The latter has contributed greatly towards the disproportionate increase in the human brain size and its higher susceptibility to AD. Indeed, during recent past four million years of evolution, the ~400 cm3 brain of human ancestors has transformed into the ~ 1465 cm3 brain of the Homo sapiens, primarily owing to the enlargement of cortical structures, and more specifically, the association areas [10–12] (Fig. 1). Although the evolution of the association cortex in human ancestors has been largely allometric, the process involved a major reorganization of connected cortical and subcortical structures, in accordance with the model of integrated phylogeny [12–14]. As a result of this process, basal forebrain (BF) cholinergic nuclei became more specialized and compartmentalized in primates, and especially in humans [15–17]. Changes in BF nuclei, however, have been relatively modest, as compared to the major expansion of the association cortex [15,18–22]. Such asymmetric upgrades of the deeply integrated sub-systems of the brain, therefore, entailed a selective pressure for elaboration of basalo-cortical projections, to ensure acetylcholine supply to expanding innervation fields, resulting in a rise in functional and metabolic load of source neurons.
Fig. 1.
Enlargement of human brain capacity (A) and increase in average life expectancy of the Homo sapiens throughout ~ 4 million years of evolution (B).
As discussed in this study, the radical expansion of the association structures with a conserved BF seem to play a key role in the selective vulnerability of the human brain to amyloid β (Aβ) depositions and related pathological alterations of AD. Indeed, unlike the vast majority of sporadic cases exhibiting complex and characteristic amyloid plaques and neurofibrillary tangles with widespread loss of synapses and neurodegeneration, combination of these changes are extremely rare in other mammals, including non-human primates [23,24]. To date, Aβ deposits have been described in a few occasions in camels, polar bears, dogs, and cats, with neuritic plaques and vascular deposits also present [25–28]. Importantly, in all these animals, the amyloid pathology and neurodegeneration occur spontaneously, unlike laboratory rodents, where they occur as a result of genetic manipulations. In aged dogs, for instance, while extracellular Aβ (oligomers and fibrils) deposits have been demonstrated, the dog brain rarely contains neurofibrillary tangles [29]. Aged dogs also display signs of vasculopathies with degeneration of cholinergic neurons of the BF, with the latter not correlating with the extent of the Aβ cortical load [29,30]. Research by Raghanti and co-workers have demonstrated several pathological hallmarks of AD in aged chimpanzee, including parenchymal and vascular amyloid deposits [31–34]. Authors also report AD-like changes in microglia phenotypes with increased activation, which unlike human AD cases, did not correlate with neurofibrillary tangle pathology [33]. Likewise, although chimpanzees exhibit age-related regional neuronal loss, they appear protected from cell death found in AD [31]. Overall, in non-human primates as well as in other animals, the extent and intensity of amyloid pathology with neurofibrillary tangles and related neuropathological effects vary considerably and are being less robust as compared to human AD. These and a growing number of similar studies have recently led to the notion that while some AD-like signs can manifest in aging primates and other mammals, only humans develop the fully blown neuropathological and clinical phenotype of the disease.
In developing the thesis of AD as a human-specific condition, Rapoport and others noted that one of the corollary criteria for qualifying it as such is that the pathology affects evolutionary most recent and rapidly expanding cortical structures, which arose during primate and especially human speciation [7,8,35]. Although comparative evidence supports the selective vulnerability of specific cortical areas and functions to the disease, the underlying mechanisms of such effects remain controversial. Proposed mechanistic models view pathological alterations in the AD cortex exclusively as a fall-out of intrinsic processes [2, 7,36], neglecting the potential contribution of subcortical effects. Given the well-recognized early onset deficiency of acetylcholine (ACh) with loss of cortical cholinergic innervations in AD, none of the proposed models so far can be considered as complete, but warrant conciliatory revision. Throughout this study, we provide a unifying outlook at AD evolution that integrates established and more recently emerging facets of the neurobiology of the BF cholinergic system along with their potential contribution to greater susceptibility of the expanding human cortex to the disease.
2. Differential susceptibility of cortical regions to AD pathology
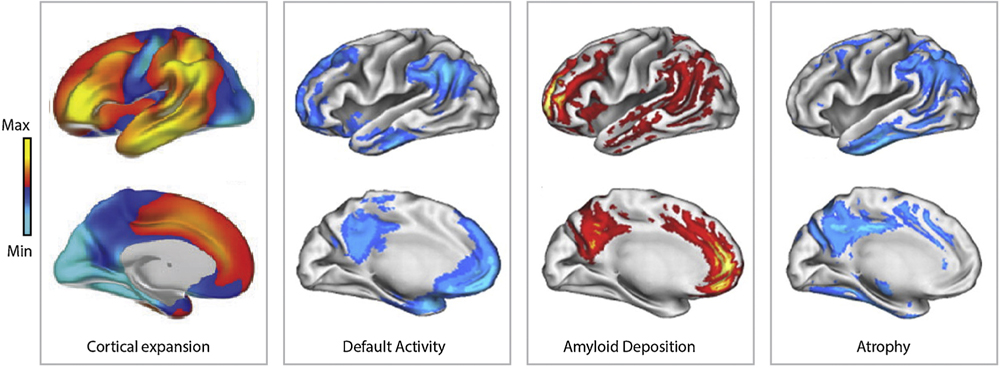
There is overwhelming data from functional brain imaging and autopsy studies suggesting differential vulnerability of various cortical regions to neuropathological changes of AD. In the majority of reports, the onset of cortical pathology with early amyloid-related impairments have been mapped to temporal areas, spreading from there over the medial parietal, prefrontal, posterior parietal association areas, orbitofrontal cortex, and cingulate gyrus [37–40]. At more advanced stages of the disease, the neuropathological changes propagate further over the primary sensory and motor areas as well as subcortical structures. Such orderly spread of the pathology provided a basis for staging of AD proposed by Braak and co-workers [37]. Mapping and correlational of cortical regions affected by AD with networks of resting-state activity, or so-called default mode network (i.e. DMN), has shown a high degree of spatial correspondence [41,42] (Fig. 2), which has been interpreted as evidence for a mechanistic connection between neuronal activity and amyloid load with neurotoxicity [41,43,44]. Importantly, DMNs also overlap with evolutionary youngest and most dynamic cortical regions. Despite growing data supporting the link between neuronal activity with the release of the Aβ peptide from neurons and neuronal activity, a major gap remains in understanding specific mechanisms of the higher susceptibility of the evolutions most progressive and plastic cortical structures to the disease [36]. The dependence of APP processing and Aβ production on synaptic activity might contribute towards greater amyloid load [45–48] given the above average basal activity in DMN circuits [42,49]. Anatomical evidence shows that dramatic evolutionary enlargement of temporal association, prefrontal, and parietal areas was related to the reorganization of connectivity, to process increasingly complex and multimodal information related to sensory inputs and intrinsic cortical activity. Accordingly, pyramidal cells in human association areas show more elaborate synaptic architecture with complex connections, as compared to those in other cortical regions in humans and non-human primates [40,50,51].
Fig. 2.
Demonstration of topographical relationship between cortical regions that underwent significant expansion throughout human evolution (left) with those known as default mode networks (left middle), areas with the highest load of amyloid β peptide (right middle) and undergoing atrophy during AD (right). Adapted with permission [8,55].
The fundamental link between the level of neuronal activity and metabolism with production of toxic by-products is also in agreement with early-onset neurodegeneration and atrophy of selected association areas, given the reliance of neurons and glial cells on aerobic metabolism, with related risks for oxidative damage [52,53]. Accordingly, the decrease in glucose metabolism (hypo-metabolism) has been reported from the early stage of AD, with affected areas closely overlapping with the most active cortical regions [49,54,55]. Moreover, it was shown that the association circuits with above-average activity maintain large number of juvenile neurons, displaying higher structural plasticity with incomplete myelination [2,56–58]. The latter is expected to contribute to greater energy demands with higher risks for oxidative stress and toxicity. Indeed, during development, parts of the prefrontal cortex and parietal association areas display the slowest myelination, with a large fraction of axons maintained in non-myelinated state in adulthood. The combination of higher activity levels of juvenile neurons with low levels of myelination, hence, rear the energy expenditure and related risk of oxidative damage an above average level [58]. Also, the high concentration of iron in immature oligodendrocytes, which is released upon their damage, would promote Aβ oligomerization with plaque formation.
Arendt and co-workers proposed that the greater vulnerability of the association cortex to AD could also be contributed by the excessive rounds of division of neuronal precursors supplying neurons toexpanding cortical regions [7]. Although the general mechanisms of cortical development in mammals are relatively conserved, major variations in cortical size in different lineages implies considerable evolutionary adjustments in cell proliferation programs, resulting in a differential supply of neurons. The expanding cortex, thus, requires a supply of extra neurons produced via additional mitotic rounds [59–62]. Generated by asymmetric division, post-mitotic neurons migrate out of the ventricular area, leaving behind dividing progenitors. Analysis of the kinetics of cell division in monkeys and murine models [59,60] showed that in macaques, the period during which progenitor cells proliferate is 10 times longer than in mice. The extended cell division accounts for the expansion of the cortical sheet, including in primates and humans. Unlike progenitor cells in the ventricular zone of mice undergoing ~11 rounds of division, in macaque, progenitors of cortical neurons divide at least 28 times, with these numbers most likely even higher in humans [59,60,62–64]. Additional mitotic cycles of progenitors, hence, increases the exposure of new cells to harmful exogenous and endogenous factors, resulting in genetic aberrations, which accumulate, enhancing the rate of molecular errors and propensity to degeneration.
While supported by strong experimental data, the principal shortfall of the discussed models is that they attribute the cause of selective susceptibility of the association cortex to AD exclusively to intrinsic cortical processes, disregarding subcortical effects. Also, none of the models explains the early onset of ACh deficit with loss of cholinergic innervations, which is one of the best recognized characteristic of the disease. Finally, all models leave unattended the potential contribution of a reduced functional reserve of the BF cholinergic system, due to an out of proportion expansion of cortical projection fields.
3. Integrated evolution of the human brain and AD
There has been a long-standing debate as to whether the cephalization process in mammals has gone through a mosaic reorganization of selected brain structures, or evolutionary upgrades occurred as an integral part of a holistic process [14]. Comparative anatomical evidence suggests that dramatic expansion of association areas in the course of mammalians brain evolution is a part of a broader process [10,12,14]. Neuro-anatomical analysis with connectome studies have shown that as the size of the forebrain has increased, largely due to the growth of frontal, occipital, and parietal association areas, other connected structures also underwent considerable reorganization [12,14]. In primates, for instance, the enlargement of the neocortex was paralleled with upgrades in old cortical structures such as the hippocampus and particularly the CA2 and subicular regions with associated entorhinal area [65,66]. Similar reorganisations occurred in subcortical formations, including BF cholinergic nuclei, which are known to play a key role in modulating functions and plasticity of association circuits [12,16, 17,67–70]. In primates and humans, for instance, changes in the size and complexity of the medial septum and nucleus basalis Meynert (NBM) show a clear connection with upgrades and expansion of the cerebral cortex [12,17]. This trend is evident from the relatively early stages of mammalian evolution. In insectivores with the rudimentary cortex, the cholinergic BF is not discernible. In rodents, the cholinergic BF nuclei can be readily distinguished, although they remain somewhat underdeveloped and incompletely demarcated from the adjacent globus pallidus [71,72]. In macaques and in higher primates, the cholinergic BF is well developed and shows features of a high compartmentalization. In humans, this evolutionary trend led to condensation of BF cholinergic cells in elaborate chain of nuclei, with NBM reaching its highest organization, comprised of densely packed neurons readily discernible from the overlying globus pallidus [17]. The resulting human NBM presents sole source of cortical cholinergic innervations comprised of ~200 000 neurons within each hemisphere which project their axons free of long collaterals to the cerebral cortex, innervating specific cortical fields [73, 74].
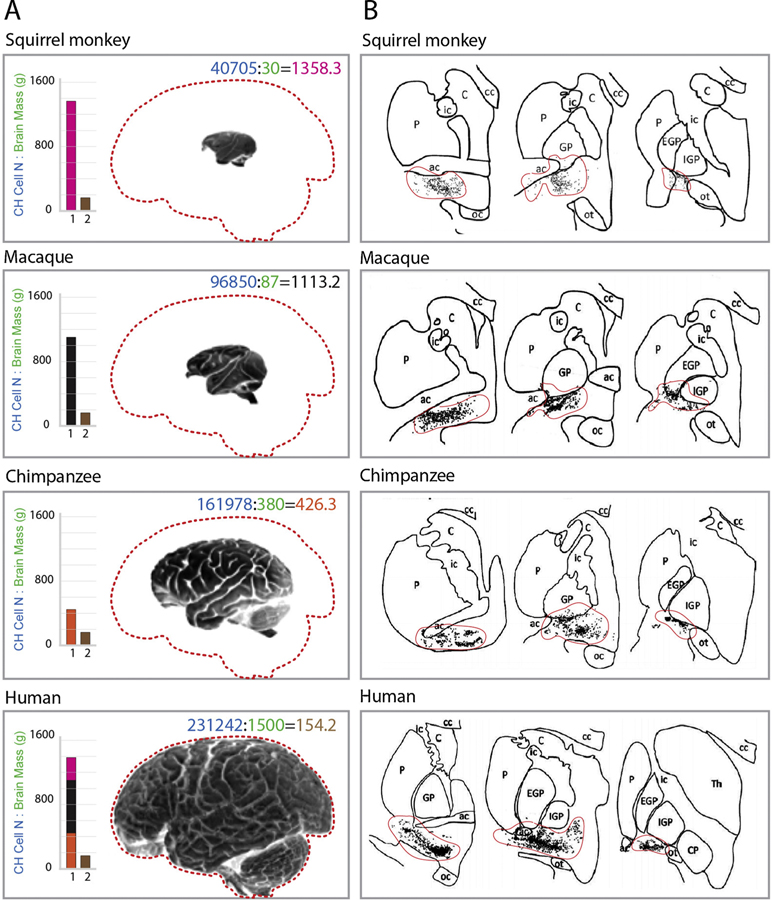
As already noted, upgrades of the BF cholinergic system were far modest as compared to those in cerebral cortex (Fig. 3). Indeed, the radical enlargement of the neocortex in humans resulted in a structure ~5000 times larger than that in mice, ~1400 times than that in rats, and ~4 times than that of our closest relative chimpanzee. For comparison, increase in the number of BF cholinergic cells in humans was only ~100–150 times as compared to mice and rats, respectively, and less than ~40 % as compared to chimpanzee [15,18–21]. Notably, the cortical expansion in primates and humans was associated with reinforcement of cholinergic innervations, with density of cholinergic axons in humans ~4–6 times higher than that in rat and mouse, and ~10–15% higher than that in chimpanzee [15,21]. In the absence of cortical cholinergic neurons in humans, the disproportionate enlargement of the associative fields with increasing cholinergic innervations infers a major elaboration of cholinergic axons [18]. Given that the increase in axon size comes with considerable neurobiological challenges [75,76], it is reasonable to think that the most recent evolutionary upgrades of basalo-cortical cholinergic projections with cortical expansion might have contributed towards the selective vulnerability of the human brain to AD. If nothing else, larger axons present a major source of cellular expenditure as well as challenges for transport of trophic factors and metabolites, exposing neurons to greater risks [77,78].
Fig. 3.
Brain size changes in primates and humans with relatively conserved BF cholinergic nuclei. (A) From top to bottom: illustration photos of brains of the squirrel monkey, macaque, chimpanzee, and human with outlines (dashed, red) of the human brain for better visibility of size differences. Inset histograms illustrate the relative ratio of cholinergic (CH) cell number and brain mass of squirrel monkey (rhodamine red bar), macaque (black bar), and chimpanzee (orange bar) vs. humans (all three superimposed, left bar). Brown bars on the right in all histograms show the ratio of CH cells and brain mass of humans. Note that the ratio of CH cell number and brain mass is the lowest in humans, indicating the lowest number of cholinergic neurons per innervation cortical area. Numbers in panels: blue – cholinergic cell number in NBM, green – the mass of the brain in grams. (B) Schematic of coronal brain sections containing cholinergic forebrain area (black dots, delignated by a red line) illustrating relative conservation of cholinergic nuclei from squirrel monkey to human. Ot - optic tract; ac – anterior commissure; EGP and IGP – external and internal globus pallidus; Th – thalamus; ic – internal capsule; cc – corpus callosum; C – claustrum; P – putamen; Adapted with permission [15].
In light of all mentioned, early-onset acetylcholine decline in the AD cortex followed by depletion of cholinergic innervations might present a knock-on effect of unbalanced evolutionary expansion and upgrades of cholinergic BF structures and projection association fields. Of note, the degeneration of cholinergic axons in the cortex adheres to a considerable anatomical specificity, with association areas typically showing strong depletion of cholinergic innervations, while cholinergic innervations of motor and anterior cingulate cortices, and sensory areas remain relatively intact [40,67]. In functional brain imaging studies, there is a significant overlap between the DMN and cortical regions undergoing extensive depletion of cholinergic innervations of AD, with ~45–85% of ChAT-positive fibre degeneration reported in temporal, prefrontal, posterior parietal, and orbitofrontal areas and cingulated gyrus, whereas cholinergic inputs to primary sensory and motor sub-systems stay relatively unaffected (5 %–15 % loss of ChAT-positive axons) [79]. Although the extent of amyloid plaque load does not directly correlate with the absolute amount of degenerated cholinergic axons, it correlates significantly with the percentage of lost cholinergic axons [80]. Importantly, the regional density of residual cholinergic axons in AD seems to reflect differences in premorbid levels of cholinergic innervations, with the most severely affected cortical regions i.e. inferior, medial, and superior temporal association areas appearing almost completely denuded of cholinergic innervations. In contrast, structures with a higher premorbid density of cholinergic innervations retain higher levels of residual fibres [81]. As discussed below, along with extra metabolic and functional burden, evolutionary expansion of cholinergic axons with extended innervation fields entail considerable additional consignments related to regulation of APP processing with Aβ production, as well as control of MAP tau phosphorylation and Aβ clearance [77,78].
4. The homeostatic hypothesis of the cholinergic system and AD
As noted, despite considerable supportive evidence, the principal limitation of all models explaining the selective vulnerability of the association cortex to AD is that they assign the cause of cortical pathology to intrinsic processes. Also, none of the models explains the age-dependence of the onset of sporadic AD and degeneration of cholinergic neurons [79,82]. There is substantial evidence showing a positive correlation between the extent of cortical amyloid pathology and percentage of cholinergic axon loss [79]. With the progression of the disease, the degenerative process propagates back to the source neurons in the BF, and especially in the NBM [83]. It is important to stress that neither amyloid pathology nor cognitive decline in AD can be solely attributed to depletion of cortical ACh, as a comparable decrease in cortical ACh in subjects with olivo-ponto-cerebellar atrophy reveals neither accumulation of Aβ plaques nor AD-like cognitive deficit [84, 85]. These findings together with overtly intact striatal and brainstem cholinergic systems in the AD brain [86,87] suggest unique neurobiological characteristics of the BF cholinergic system, which extend its role beyond diffuse supply of cortical ACh and neuromodulation. In agreement with this view, Mesulam proposed that the selective loss of cortical cholinergic innervations is unlikely to be related with cholinergic mechanisms only, but may reflect the unique anatomical position and connectivity of the NBM [88].
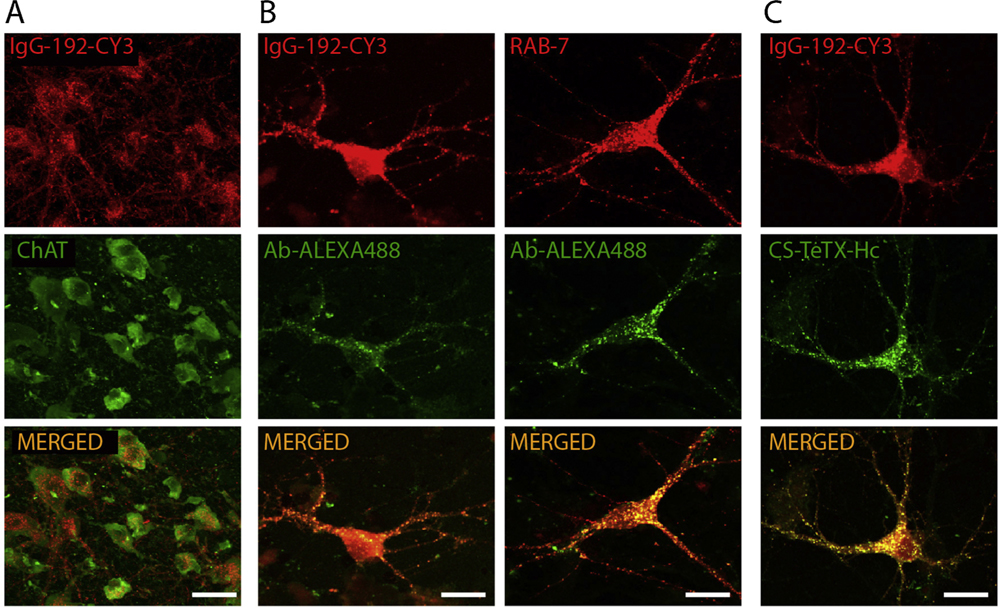
We and others have shown that in addition to widely appreciated modulator functions, cortical cholinergic innervations might play a key role in homeostatic regulation of APP processing and clearance of Aβ [89,90]. As reported by Nitsch et al. [91], activation of M1 and M3 muscarinic AChR suppresses the amyloidogenic processing of APP with Aβ production. This finding was replicated in neuronal cultures, brain slices, and in vivo, with anti-Aβ effects of M1 receptors being attributed to stimulation of protein kinase C a/ε and downregulation of α-secretase ADAM17 activity [89]. We have shown the significance of cholinergic BF neurons and their projections in the sequestration of Aβ followed by lysosomal degradation, which might be of relevance to the maintenance of physiological levels of Aβ in cortical projection fields [77,78]. Such a unique homeostatic role is attributed to cholinergic axons enriched with the p75 neurotrophin receptor (NTR), which is known to bind and facilitate the internalization of Aβ mono- and oligomers [92], followed by degradation [77] (Fig. 4). In agreement with homeostatic role of basalo-cortical projections, selective ablation of BF cholinergic neurons or genetic deletion of p75NTR accelerates the deposition of Aβ plaques and associated histopathological changes in the cortex and hippocampus in AD murine models [93–96]. These findings accord with observations in rabbits, which showed progressive accumulation of perivascular Aβ after targeted lesion of BF cholinergic neurons with targeted immunotoxins [97]. The absence of axonal dystrophies in Thy1-hAPP-London/Swe-p75NTR−/− mice, which contrasts with widespread axonal pathology and loss of BF cholinergic cells in Thy1-hAPP-London/Swe-p75NTR+/+ genotype [98] supports the important role of p75NTR in mediating neurotoxic effects of Aβ in BF cholinergic neurons. It also suggests that the role of cholinergic inputs to the hippocampus and cortex extends beyond the supply of ACh with neuromodulator effects. Such dual functionality of cholinergic innervations agrees with results of human autopsy studies, which revealed a stronger decline in the number of p75NTR expressing BF cholinergic cells in plaque laden AD brains [99,100].
Fig. 4.
Demonstration of selective enrichment of BF cholinergic neurons with p75NTR and mediated by this receptor internalization of Aβ peptide in cholinergic neurons with sorting to acidifying late endosomes. (A) Confocal images of rat BF cholinergic area with neurons labelled using ChAT markers specific for cholinergic neurons and IgG-192-CY3 labelling p75NTR enriched neurons. Note a high degree of colocalization. Adapted with permission [103]. (B) Cholinergic neurons in primary neuronal cultures after exposure to Alexa488 labelled Aβ and IgG-192-CY3 (left) and staining with an anti-RAB-7 antibody specific for late endosomes (right). (C) Cholinergic neurons in primary neuronal cultures after exposure to IgG-192-CY3 and core streptavidin fused with heavy chain of tetanus toxin, know also to bind to p75 NTR (CS-TeTX-Hc). Courtesy of I. Antyborzec (image taken from Ph.D. thesis).
With above average activity and higher propensity of Aβ load in the associative cortex, degeneration of p75NTR enriched cholinergic innervations is expected to further exacerbate the progression of amyloid pathology. On the other hand, the increase of Aβ level would overwhelm the proteolytic machinery of cholinergic neurons, aggravating further amyloid toxicity and lysosomal deficiency, leading to metabolic collapse and neuronal degeneration. Unlike double transgenic APPSwe/PS1dE9 mice in which selective ablation of BF cholinergic cells facilitates cortical Aβ load with cognitive decline and memory deficit [95], APPSwe/PS1dE9/p75NTR−/− triple transgenic mice reveal no cognitive decline and memory deficit, despite extensive amyloid pathology in the hippocampus and cerebral cortex [96]. Such dissociation of cognitive and homeostatic role of the BF cholinergic system supports its dual, ACh- and p75NTR-dependent functionality, and agrees with results of the above mentioned above clinical studies of olivo-ponto-cerebellar atrophies [84,85].
In addition to well-known modulator functions, BF cholinergic projections, thus, seem to play an essential homeostatic role in clearance of Aβ from projection fields, mediated via a p75NTR mechanism. Conceivably, with expansion of the association cortex and elaboration of basalo-cortical innervations in humans, cholinergic neurons came under excessive strain to maintain their dual, neuromodulator and homeostatic role. Discussed above examples of the dissociation between cognitive and homeostatic mechanisms of the BF cholinergic neurons is in agreement with their dual role, with dramatic expansion of the cortex contributing towards a reduced functional reserve and a higher vulnerability of the human brain to AD. Whereas the cognitive and mnemonic mechanisms depend on the modulation of the neuronal activity and plasticity by ACh [70,101], the homeostatic and neuroprotective effects involve regulation of APP metabolism as well as p75NTR-mediated sequestration of the Aβ peptide [78,90], keeping in check its physiological level and activity.
5. Closing remarks
Over the past four million years of human evolution, the brain of our primate ancestor quadrupled in size, resulting in the brain of the Homo sapiens. Such dramatic enlargement of the organ of thought with functional upgrades has been key to the transition of the Homo from scavenger to hunter-gatherer, which led to radical dietary changes and socio- behavioural adaptations. The restructuring of anatomical and functional landscapes of the human brain were accounted for largely by the expansion of the frontal, occipital, and parietal association fields, which evolved to meet the emerging needs and challenges of dynamic and highly complex organization of the society, leading to a remarkable extension of life expectancy (Fig. 1). Even under high mortality experienced by hunters-foragers, human life expectancy (LE0) was twice that of chimpanzee [102], which doubled further during the recent industrialization, causing a major extension in older ages with related physiological conditions and diseases.
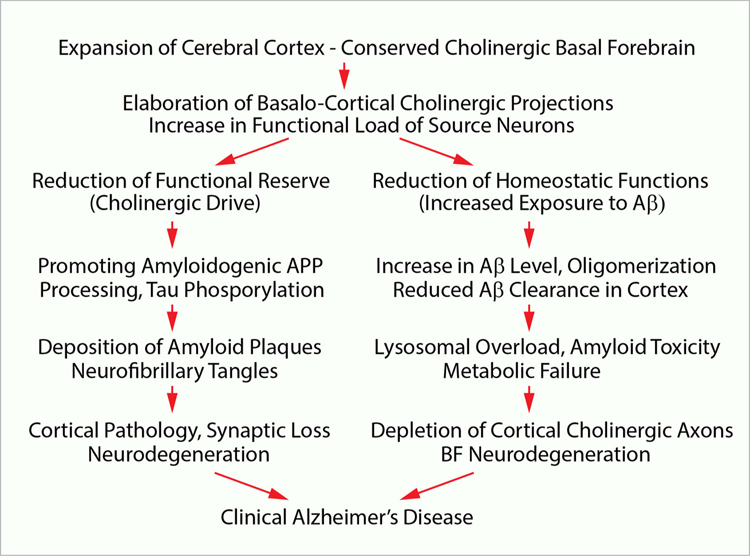
As it emerges from this review, major adaptive changes in the brain with increased LE0 came with considerable costs, conforming to J. H. Jackson’s ‘doctrine’ that evolutionary upgrades of the human nervous system might conceal neurological and psychiatric conditions, which are human-specific. Indeed, the expansion of cortical association fields with reinforcement of cholinergic innervations combined with prolongation of the LE0 has placed the BF cholinergic system under major homeostatic and metabolic strain, which possibly contributed to higher incidents of age-related degeneration of cholinergic neurons as well as sporadic amyloid pathology with degenerative changes characteristic to AD (Fig. 5). In summary, the out-of-proportion enlargement of innervation fields of individual cholinergic neurons and LE0 extension lowered the functional reserve of the BF cholinergic system and enhanced the homeostatic assignments in terms of Aβ clearance and regulation of APP metabolism, overwhelming proteolytic and metabolic machinery of cholinergic neurons, which have led to higher risks of amyloid pathology and age-dependent onset of AD, with functional impairments and neurodegeneration. While bearing an array of major adaptive benefits and protective effects empowering progressive mental facilities, the disproportionate growth of the human cerebral cortex, thus, came with significant unfavourable effects, with implications for the neurobiology of aging and global health.
Fig. 5.
Schematic illustration of proposed sequence of events leading to sporadic AD manifested by depletion of cholinergic innervations with loss in the basal forebrain cholinergic neurons, and the onset of amyloid pathology with neurodegeneration in the cerebral cortex.
Acknowledgements
This study was supported by the project Sustainability for the National Institute of Mental Health, under grant number LO1611 (PI – CH), with financial support from the Ministry of Education, Youth and Sports of the Czech Republic under the NPU I program. Dr. V. B. O’Leary is supported by the Charles University research program PROGRES Q35 – Neurology.
References
- [1].Jackson JH, The Croonian Lectures on Evolution and Dissolution of the Nervous System, Br. Med. J. 1 (1884) 591–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bufill E, Blesa R, Augusti J, Alzheimer’s disease: an evolutionary approach, J. Anthropol. Sci. 91 (2013) 135–157. [DOI] [PubMed] [Google Scholar]
- [3].van den Heuvel MP, Scholtens LH, de Lange SC, Pijnenburg R, Cahn W, van Haren NEM, Sommer IE, Bozzali M, Koch K, Boks MP, Repple J, Pievani M, Li L, Preuss TM, Rilling JK, Evolutionary modifications in human brain connectivity associated with schizophrenia, Brain 142 (2019) 3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walker LC, Jucker M, The Exceptional Vulnerability of Humans to Alzheimer’s Disease, Trends Mol. Med. 23 (2017) 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].2020 Alzheimer’s disease facts and figures, Alzheimers Dement, DOI 10.1002/alz.12068(2020). [DOI] [PubMed] [Google Scholar]
- [6].Collaborators GBDN, Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016, Lancet Neurol. 18 (2019) 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arendt T, Stieler J, Ueberham U, Is sporadic Alzheimer’s disease a developmental disorder? J. Neurochem. 143 (2017) 396–408. [DOI] [PubMed] [Google Scholar]
- [8].Fjell AM, Amlien IK, Sneve MH, Grydeland H, Tamnes CK, Chaplin TA, Rosa MG, Walhovd KB, The roots of alzheimer’s disease: are high-expanding cortical areas preferentially targeted? Dagger Cereb Cortex 25 (2015) 2556–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Krienen FM, Buckner RL, Human association cortex: expanded, untethered, neotenous, and plastic, in: Kaas JH (Ed.), Evolutionary Neuroscience, Academic Press, 2020, pp. 845–860. [Google Scholar]
- [10].Hofman MA, Evolution of the human brain: when bigger is better, Front. Neuroanat 8 (2014) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hofman MA, On the nature and evolution of the human mind, Prog. Brain Res. 250 (2019) 251–283. [DOI] [PubMed] [Google Scholar]
- [12].Rapoport SI, Integrated phylogeny of the primate brain, with special reference to humans and their diseases, Brain Res. Brain Res. Rev. 15 (1990) 267–294. [DOI] [PubMed] [Google Scholar]
- [13].Kaas JH, The origin and evolution of neocortex: From early mammals to modern humans, Prog. Brain Res. 250 (2019) 61–81. [DOI] [PubMed] [Google Scholar]
- [14].Striedter GF, Principles of Brain Evolution, Sinauer Associates, Sunderland, Mass, 2005. [Google Scholar]
- [15].Raghanti MA, Simic G, Watson S, Stimpson CD, Hof PR, Sherwood CC, Comparative analysis of the nucleus basalis of Meynert among primates, Neuroscience 184 (2011) 1–15. [DOI] [PubMed] [Google Scholar]
- [16].Semba K, Phylogenetic and ontogenetic aspects of the basal forebrain cholinergic neurons and their innervation of the cerebral cortex, Prog. Brain Res. 145 (2004) 3–43. [DOI] [PubMed] [Google Scholar]
- [17].Gorry JD, Studies on the Comparative Anatomy of the Ganglion Basale of Meynert, Acta Anat. (Basel) 55 (1963) 51–104. [DOI] [PubMed] [Google Scholar]
- [18].Wu H, Williams J, Nathans J, Complete morphologies of basal forebrain cholinergic neurons in the mouse, Elife 3 (2014), e02444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Perez SE, Dar S, Ikonomovic MD, DeKosky ST, Mufson EJ, Cholinergic forebrain degeneration in the APPswe/PS1DeltaE9 transgenic mouse, Neurobiol. Dis. 28 (2007) 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Boncristiano S, Calhoun ME, Kelly PH, Pfeifer M, Bondolfi L, Stalder M, Phinney AL, Abramowski D, Sturchler-Pierrat C, Enz A, Sommer B, Staufenbiel M, Jucker M, Cholinergic changes in the APP23 transgenic mouse model of cerebral amyloidosis, J. Neurosci. 22 (2002) 3234–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miettinen RA, Kalesnykas G, Koivisto EH, Estimation of the total number of cholinergic neurons containing estrogen receptor-alpha in the rat basal forebrain, J. Histochem. Cytochem. 50 (2002) 891–902. [DOI] [PubMed] [Google Scholar]
- [22].Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC, Cholinergic innervation of the frontal cortex: differences among humans, chimpanzees, and macaque monkeys, J. Comp. Neurol. 506 (2008) 409–424. [DOI] [PubMed] [Google Scholar]
- [23].Youssef SA, Capucchio MT, Rofina JE, Chambers JK, Uchida K, Nakayama H, Head E, Pathology of the aging brain in domestic and laboratory animals, and animal models of human neurodegenerative diseases, Vet. Pathol. 53 (2016) 327–348. [DOI] [PubMed] [Google Scholar]
- [24].Gunn-Moore D, Kaidanovich-Beilin O, Gallego Iradi MC, Gunn-Moore F, Lovestone S, Alzheimer’s disease in humans and other animals: A consequence of postreproductive life span and longevity rather than aging, Alzheimers Dement. 14 (2018) 195–204. [DOI] [PubMed] [Google Scholar]
- [25].Perez SE, Raghanti MA, Hof PR, Kramer L, Ikonomovic MD, Lacor PN, Erwin JM, Sherwood CC, Mufson EJ, Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla), J. Comp. Neurol. 521 (2013) 4318–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Perez SE, Sherwood CC, Cranfield MR, Erwin JM, Mudakikwa A, Hof PR, Mufson EJ, Early Alzheimer’s disease-type pathology in the frontal cortex of wild mountain gorillas (Gorilla beringei beringei), Neurobiol. Aging 39 (2016) 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, Davis-Turak J, Coppola G, Geschwind DH, Pare JF, Duong TQ, Hopkins WD, Preuss TM, Walker LC, Tauopathy with paired helical filaments in an aged chimpanzee, J. Comp. Neurol. 509 (2008) 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rosen RF, Tomidokoro Y, Farberg AS, Dooyema J, Ciliax B, Preuss TM, Neubert TA, Ghiso JA, LeVine H 3rd., L.C. Walker, Comparative pathobiology of beta-amyloid and the unique susceptibility of humans to Alzheimer’s disease, Neurobiol. Aging 44 (2016) 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Prpar Mihevc S, Majdic G, Canine Cognitive Dysfunction and Alzheimer’s Disease - Two Facets of the Same Disease? Front. Neurosci. 13 (2019) 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Insua D, Corredoira A, Gonzalez-Martinez A, Suarez ML, Santamarina G, Sarasa M, Pesini P, Expression of p75(NTR), a marker for basal forebrain cholinergic neurons, in young and aged dogs with or without cognitive dysfunction syndrome, J. Alzheimers Dis. 28 (2012) 291–296. [DOI] [PubMed] [Google Scholar]
- [31].Edler MK, Munger EL, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, Sherwood CC, Raghanti MA, Neuron loss associated with age but not Alzheimer’s disease pathology in the chimpanzee brain, Philos. Trans. R. Soc. Lond., B, Biol. Sci. 375 (2020), 20190619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Edler MK, Sherwood CC, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, Raghanti MA, Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease, Neurobiol. Aging 59 (2017) 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Edler MK, Sherwood CC, Meindl RS, Munger EL, Hopkins WD, Ely JJ, Erwin JM, Perl DP, Mufson EJ, Hof PR, Raghanti MA, Microglia changes associated to Alzheimer’s disease pathology in aged chimpanzees, J. Comp. Neurol. 526 (2018) 2921–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Munger EL, Edler MK, Hopkins WD, Ely JJ, Erwin JM, Perl DP, Mufson EJ, Hof PR, Sherwood CC, Raghanti MA, Astrocytic changes with aging and Alzheimer’s disease-type pathology in chimpanzees, J. Comp. Neurol. 527 (2019) 1179–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rapoport SI, Hypothesis: Alzheimer’s disease is a phylogenetic disease, Med. Hypotheses 29 (1989) 147–150. [DOI] [PubMed] [Google Scholar]
- [36].Ovsepian SV, O’Leary VB, Neuronal activity and amyloid plaque pathology: an update, J. Alzheimers Dis. 49 (2016) 13–19. [DOI] [PubMed] [Google Scholar]
- [37].Braak H, Braak E, Neuropathological staging of Alzheimer-related changes, Acta Neuropathol. 82 (1991). [DOI] [PubMed] [Google Scholar]
- [38].Braak H, Braak E, Staging of Alzheimer-related cortical destruction, Int. Psychogeriatr 9 (Suppl 1) (1997) 257–261, discussion 269–272. [PubMed] [Google Scholar]
- [39].Thal DR, Rub U, Orantes M, Braak H, Phases of A beta-deposition in the human brain and its relevance for the development of AD, Neurology 58 (2002) 1791–1800. [DOI] [PubMed] [Google Scholar]
- [40].Mesulam MM, Aging, Alzheimer’s Disease, and Dementia: Clinical and Neurological Perspectives, Oxfor University Press, Oxford, 2000. [Google Scholar]
- [41].Buckner RL, The serendipitous discovery of the brain’s default network, Neuroimage 62 (2012) 1137–1145. [DOI] [PubMed] [Google Scholar]
- [42].Raichle ME, The brain’s default mode network, Annu. Rev. Neurosci. 38 (2015) 433–447. [DOI] [PubMed] [Google Scholar]
- [43].Jagust W, Vulnerable neural systems and the borderland of brain aging and neurodegeneration, Neuron 77 (2013) 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jagust WJ, Mormino EC, Lifespan brain activity, beta-amyloid, and Alzheimer’s disease, Trends Cogn. Sci 15 (2011) 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ovsepian SV, O’Leary VB, Zaborszky L, Ntziachristos V, Dolly JO, Synaptic vesicle cycle and amyloid beta: biting the hand that feeds, Alzheimers Dement. 14 (2018) 502–513. [DOI] [PubMed] [Google Scholar]
- [46].Yamamoto K, Tanei ZI, Hashimoto T, Wakabayashi T, Okuno H, Naka Y, Yizhar O, Fenno LE, Fukayama M, Bito H, Cirrito JR, Holtzman DM, Deisseroth K, Iwatsubo T, Chronic optogenetic activation augments abeta pathology in a mouse model of Alzheimer disease, Cell Rep. 11 (2015) 859–865. [DOI] [PubMed] [Google Scholar]
- [47].Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM, Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo, Neuron 48 (2005) 913–922. [DOI] [PubMed] [Google Scholar]
- [48].Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R, APP processing and synaptic function, Neuron 37 (2003) 925–937. [DOI] [PubMed] [Google Scholar]
- [49].Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA, Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease, J. Neurosci. 29 (2009) 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Elston GN, Benavides-Piccione R, DeFelipe J, The pyramidal cell in cognition: a comparative study in human and monkey, J. Neurosci. 21 (2001). RC163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].DeFelipe J, Farinas I, The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs, Prog. Neurobiol. 39 (1992) 563–607. [DOI] [PubMed] [Google Scholar]
- [52].Perry G, Taddeo MA, Nunomura A, Zhu X, Zenteno-Savin T, Drew KL, Shimohama S, Avila J, Castellani RJ, Smith MA, Comparative biology and pathology of oxidative stress in Alzheimer and other neurodegenerative diseases: beyond damage and response, Comp. Biochem. Physiol. C Toxicol. Pharmacol 133 (2002) 507–513. [DOI] [PubMed] [Google Scholar]
- [53].Tonnies E, Trushina E, Oxidative stress, synaptic dysfunction, and alzheimer’s disease, J. Alzheimers Dis. 57 (2017) 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Grothe MJ, Teipel SJ, Alzheimer’s Disease Neuroimaging I, Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer’s disease correspond to dissociable functional brain networks, Hum. Brain Mapp 37 (2016) 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA, Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory, J. Neurosci. 25 (2005) 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bartzokis G, Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease, Neurobiol. Aging 25 (2004) 5–18, author reply 49–62. [DOI] [PubMed] [Google Scholar]
- [57].Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J, White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study, Arch. Neurol. 60 (2003) 393–398. [DOI] [PubMed] [Google Scholar]
- [58].Bartzokis G, Lu PH, Mintz J, Human brain myelination and amyloid beta deposition in Alzheimer’s disease, Alzheimers Dement. 3 (2007) 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kornack DR, Neurogenesis and the evolution of cortical diversity: mode, tempo, and partitioning during development and persistence in adulthood, Brain Behav. Evol 55 (2000) 336–344. [DOI] [PubMed] [Google Scholar]
- [60].Kornack DR, Rakic P, Changes in cell-cycle kinetics during the development and evolution of primate neocortex, Proc. Natl. Acad. Sci. U. S. A. 95 (1998) 1242–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rakic P, Specification of cerebral cortical areas, Science 241 (1988) 170–176. [DOI] [PubMed] [Google Scholar]
- [62].Rakic P, Radial unit hypothesis of neocortical expansion, Novartis Found. Symp. 228 (2000) 30–42, discussion 42–52. [DOI] [PubMed] [Google Scholar]
- [63].Takahashi T, Nowakowski RS, Caviness VS Jr., The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall, J. Neurosci. 15 (1995) 6046–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hill RS, Walsh CA, Molecular insights into human brain evolution, Nature 437 (2005) 64–67. [DOI] [PubMed] [Google Scholar]
- [65].Herzog AG, Van Hoesen GW, Temporal neocortical afferent connections to the amygdala in the rhesus monkey, Brain Res. 115 (1976) 57–69. [DOI] [PubMed] [Google Scholar]
- [66].Stephan H, Andy OJ, The Allocortex in Primates, Appleton-Century-Crofts, New York, 1970. [Google Scholar]
- [67].Geula C, Mesulam MM, Cortical cholinergic fibers in aging and Alzheimer’s disease: a morphometric study, Neuroscience 33 (1989) 469–481. [DOI] [PubMed] [Google Scholar]
- [68].Ovsepian SV, Enhancement of the synchronized firing of CA1 pyramidal cells by medial septum preconditioning: time-dependent involvement of muscarinic cholinoceptors and GABAB receptors, Neurosci. Lett. 393 (2006) 1–6. [DOI] [PubMed] [Google Scholar]
- [69].Ovsepian SV, Differential cholinergic modulation of synaptic encoding and gain control mechanisms in rat hippocampus, Neurosci. Res. 61 (2008) 92–98. [DOI] [PubMed] [Google Scholar]
- [70].Ovsepian SV, Anwyl R, Rowan MJ, Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: in vivo study, Eur. J. Neurosci. 20 (2004) 1267–1275. [DOI] [PubMed] [Google Scholar]
- [71].Braak H, Pigment architecture of the human telencephalic cortex, Cell Tissue Res. 190 (1978) 509–523. [DOI] [PubMed] [Google Scholar]
- [72].Mesulam MM, Mufson EJ, Levey AI, Wainer BH, Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey, J. Comp. Neurol. 214 (1983) 170–197. [DOI] [PubMed] [Google Scholar]
- [73].Richardson RT, DeLong MR, A reappraisal of the functions of the nucleus basalis of Meynert, Trends Neurosci. 11 (1988) 264–267. [DOI] [PubMed] [Google Scholar]
- [74].Arendt T, Bigl V, Tennstedt A, Arendt A, Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer’s disease, Neuroscience 14 (1985) 1–14. [DOI] [PubMed] [Google Scholar]
- [75].Cavanagh JB, The problems of neurons with long axons, Lancet 1 (1984) 1284–1287. [DOI] [PubMed] [Google Scholar]
- [76].Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ, Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis, Nat. Rev. Neurol 7 (2011) 616–630. [DOI] [PubMed] [Google Scholar]
- [77].Ovsepian SV, Antyborzec I, O’Leary VB, Zaborszky L, Herms J, Oliver Dolly J, Neurotrophin receptor p75 mediates the uptake of the amyloid beta (Abeta) peptide, guiding it to lysosomes for degradation in basal forebrain cholinergic neurons, Brain Struct. Funct 219 (2014) 1527–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ovsepian SV, Herms J, Cholinergic neurons-keeping check on amyloid beta in the cerebral cortex, Front. Cell. Neurosci 7 (2013) 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mesulam M, The cholinergic lesion of Alzheimer’s disease: pivotal factor or side show? Learn. Mem. 11 (2004) 43–49. [DOI] [PubMed] [Google Scholar]
- [80].Geula C, Mesulam MM, Saroff DM, Wu CK, Relationship between plaques, tangles, and loss of cortical cholinergic fibers in Alzheimer disease, J. Neuropathol. Exp. Neurol. 57 (1998) 63–75. [DOI] [PubMed] [Google Scholar]
- [81].Geula C, Greenberg BD, Mesulam MM, Cholinesterase activity in the plaques, tangles and angiopathy of Alzheimer’s disease does not emanate from amyloid, Brain Res. 644 (1994) 327–330. [DOI] [PubMed] [Google Scholar]
- [82].Schliebs R, Arendt T, The cholinergic system in aging and neuronal degeneration, Behav. Brain Res. 221 (2011) 555–563. [DOI] [PubMed] [Google Scholar]
- [83].Mesulam MM, Geula C, Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase, J. Comp. Neurol. 275 (1988) 216–240. [DOI] [PubMed] [Google Scholar]
- [84].Kish SJ, Robitaille Y, el-Awar M, Deck JH, Simmons J, Schut L, Chang LJ, DiStefano L, Freedman M, Non-Alzheimer-type pattern of brain cholineacetyltransferase reduction in dominantly inherited olivopontocerebellar atrophy, Ann. Neurol. 26 (1989) 362–367. [DOI] [PubMed] [Google Scholar]
- [85].Robitaille Y, Schut L, Kish SJ, Structural and immunocytochemical features of olivopontocerebellar atrophy caused by the spinocerebellar ataxia type 1 (SCA-1) mutation define a unique phenotype, Acta Neuropathol. 90 (1995) 572–581. [DOI] [PubMed] [Google Scholar]
- [86].McKinney M, Jacksonville MC, Brain cholinergic vulnerability: relevance to behavior and disease, Biochem. Pharmacol. 70 (2005) 1115–1124. [DOI] [PubMed] [Google Scholar]
- [87].Woolf NJ, Jacobs RW, Butcher LL, The pontomesencephalotegmental cholinergic system does not degenerate in Alzheimer’s disease, Neurosci. Lett. 96 (1989) 277–282. [DOI] [PubMed] [Google Scholar]
- [88].Mesulam M, Cholinergic aspects of aging and Alzheimer’s disease, Biol. Psychiatry 71 (2012) 760–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fisher A, Cholinergic modulation of amyloid precursor protein processing with emphasis on M1 muscarinic receptor: perspectives and challenges in treatment of Alzheimer’s disease, J. Neurochem. 120 (Suppl 1) (2012) 22–33. [DOI] [PubMed] [Google Scholar]
- [90].Ovsepian SV, O’Leary VB, Zaborszky L, Cholinergic mechanisms in the cerebral cortex: beyond synaptic transmission, Neuroscientist 22 (2016) 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Nitsch RM, Slack BE, Wurtman RJ, Growdon JH, Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors, Science 258 (1992) 304–307. [DOI] [PubMed] [Google Scholar]
- [92].Yaar M, Zhai S, Pilch PF, Doyle SM, Eisenhauer PB, Fine RE, Gilchrest BA, Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer’s disease, J. Clin. Invest. 100 (1997) 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gil-Bea FJ, Gerenu G, Aisa B, Kirazov LP, Schliebs R, Ramirez MJ, Cholinergic denervation exacerbates amyloid pathology and induces hippocampal atrophy in Tg2576 mice, Neurobiol. Dis. 48 (2012) 439–446. [DOI] [PubMed] [Google Scholar]
- [94].Hartig W, Saul A, Kacza J, Grosche J, Goldhammer S, Michalski D, Wirths O, Immunolesion-induced loss of cholinergic projection neurones promotes beta-amyloidosis and tau hyperphosphorylation in the hippocampus of triple-transgenic mice, Neuropathol. Appl. Neurobiol 40 (2014) 106–120. [DOI] [PubMed] [Google Scholar]
- [95].Laursen B, Mork A, Plath N, Kristiansen U, Bastlund JF, Cholinergic degeneration is associated with increased plaque deposition and cognitive impairment in APPswe/PS1dE9 mice, Behav. Brain Res. 240 (2013) 146–152. [DOI] [PubMed] [Google Scholar]
- [96].Wang YJ, Wang X, Lu JJ, Li QX, Gao CY, Liu XH, Sun Y, Yang M, Lim Y, Evin G, Zhong JH, Masters C, Zhou XF, p75NTR regulates Abeta deposition by increasing Abeta production but inhibiting Abeta aggregation with its extracellular domain, J. Neurosci. 31 (2011) 2292–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Roher AE, Kuo YM, Potter PE, Emmerling MR, Durham RA, Walker DG, Sue LI, Honer WG, Beach TG, Cortical cholinergic denervation elicits vascular A beta deposition, Ann. N. Y. Acad. Sci. 903 (2000) 366–373. [DOI] [PubMed] [Google Scholar]
- [98].Knowles JK, Rajadas J, Nguyen TV, Yang T, LeMieux MC, Vander Griend L, Ishikawa C, Massa SM, Wyss-Coray T, Longo FM, The p75 neurotrophin receptor promotes amyloid-beta(1–42)-induced neuritic dystrophy in vitro and in vivo, J. Neurosci. 29 (2009) 10627–10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Counts SE, Mufson EJ, The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease, J. Neuropathol. Exp. Neurol. 64 (2005) 263–272. [DOI] [PubMed] [Google Scholar]
- [100].Qian L, Milne MR, Shepheard S, Rogers ML, Medeiros R, Coulson EJ, Removal of p75 Neurotrophin Receptor Expression from Cholinergic Basal Forebrain Neurons Reduces Amyloid-beta Plaque Deposition and Cognitive Impairment in Aged APP/PS1 Mice, Mol. Neurobiol. 56 (2019) 4639–4652. [DOI] [PubMed] [Google Scholar]
- [101].Hasselmo ME, Barkai E, Cholinergic modulation of activity-dependent synaptic plasticity in the piriform cortex and associative memory function in a network biophysical simulation, J. Neurosci. 15 (1995) 6592–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Finch CE, Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition, Proc Natl Acad Sci U S A 107 (Suppl 1) (2010) 1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Antyborzec I, O’Leary VB, Dolly JO, Ovsepian SV, Low-Affinity Neurotrophin Receptor p75 Promotes the Transduction of Targeted Lentiviral Vectors to Cholinergic Neurons of Rat Basal Forebrain, Neurotherapeutics 13 (2016) 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]