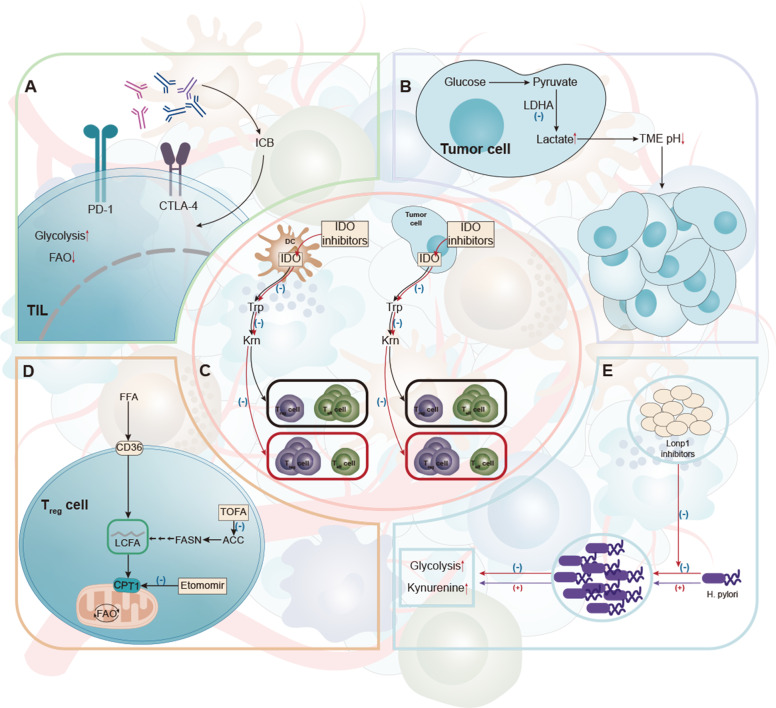
Fig. 3. Therapies that targeting immune metabolism.
A Increased checkpoint receptor expression is usually a result of low glucose, acidity, or lactic acid in the TME, and the involvement of these receptors leads to immunosuppressive phenotypic characteristics of reduced glycolysis and increased FAO. Antibodies against these checkpoint receptors have successfully restored glycolysis, which in turn supports the function of antitumor effectors within immune cells. B LDHA is required for the conversion of pyruvate to lactic acid and is associated with the development, maintenance, and metastasis of cancer. LDHA is a potential therapeutic target. C Inhibitory immune cells, including Treg, TADCs and MDSC, increased the expression of IDO, an enzyme responsible for metabolizing tryptophan into canine urine. IDO-targeting activity inhibits these suppressive immune cells in the tumor. D Immunosuppressive Treg cells often use FAO as a way to produce energy. The metabolism of Treg can be improved by using ACC inhibitors, CPT1 inhibitors or CD36 inhibitors, providing potential targets for the treatment of gastric cancer. E In the TME of specific gastric cancer, the microflora was changed. In gastric cancer patients, a large increase of H. pylori led to an increase in tumor cell glycolysis and Lonp1. Downregulation of Lonp1 expression significantly reduced glycolytic metabolic transformation and gastric cell proliferation associated with low diversity of H. pylori infection.