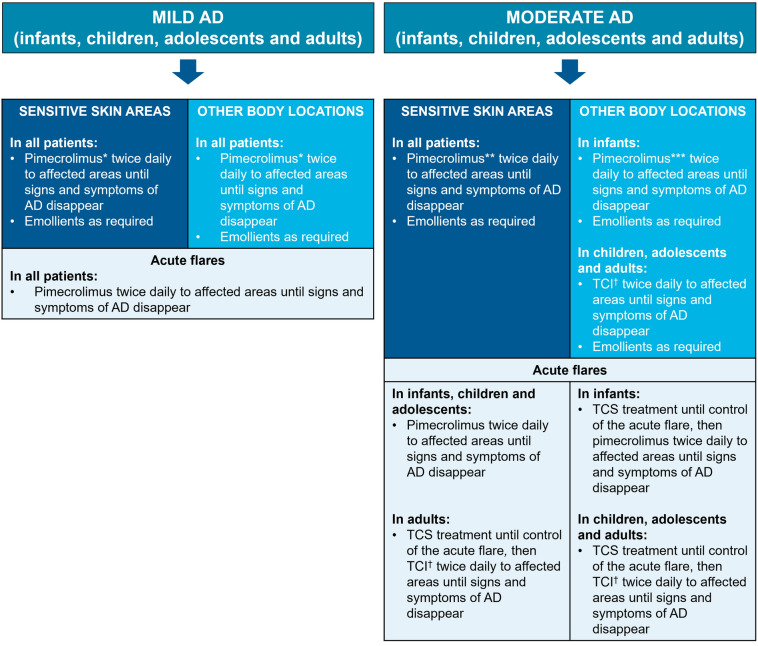
Fig. 1.
Algorithm for the treatment of infants, children, adolescents, and adults with mild-to-moderate AD. AD atopic dermatitis, EU European Union, TCI topical calcineurin inhibitors, TCS topical corticosteroids. *Pimecrolimus 1% cream is indicated for mild-to-moderate AD (children aged ≥ 2 years, adolescents, and adults) [53] and for use in infants aged ≥ 3 months (Australia, Brazil, Canada, European Union, India, Indonesia, Israel, New Zealand, Philippines, Russia, Taiwan, and Thailand only) [55–66]. **Pimecrolimus is recommended in EU guidelines [19] in sensitive skin areas; evidence suggests patient preference for pimecrolimus versus tacrolimus [100]. ***Pimecrolimus is recommended in other body locations versus tacrolimus, as there is a body of evidence to support its efficacy and tolerability profile. †TCI: pimecrolimus 1% cream, or tacrolimus 0.1% (aged ≥ 16 years) or 0.03% (aged 2–15 years) ointment; pimecrolimus is indicated for mild-to-moderate AD, and tacrolimus is indicated for moderate-to-severe AD [53, 54]