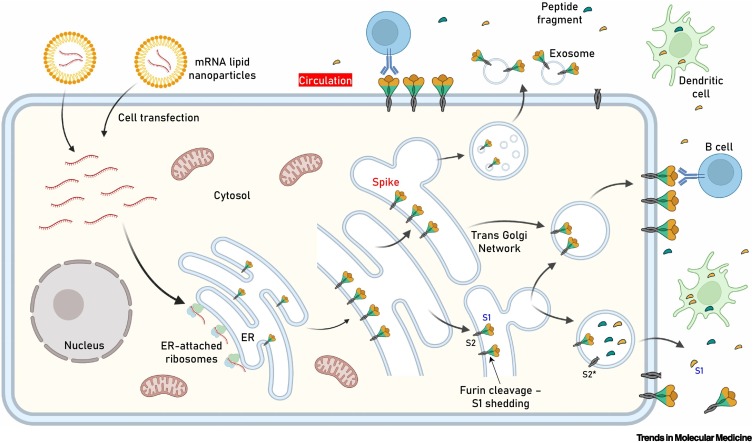
Figure 1.
Key figure. Antigen expression–localization following cell transfection with spike (S) protein mRNA-containing lipid nanoparticles (LNPs) used in anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines.
Following LNP internalization and mRNA release, the authentic viral signal peptide (as in the Pfizer–BioNTech and Moderna vaccines) drives antigen production in the lumen of the endoplasmic reticulum (ER) where it adopts its natural transmembrane localization via subunit 2 (S2) anchoring. After sorting in the trans Golgi network (TGN), S protein acquires its final position in the transfected human cell membrane, where S1 is exposed to the extracellular space (i.e., may face circulation). Although the extent of antigen expression per cell remains unknown, it is reasonable to assume that this process results in rather extended decoration of transfected cells with S protein. Furin-mediated proteolytic cleavage (as in SARS-CoV-2-infected cells) in the absence of a mutated S1/S2 furin cleavage site at the TGN may result in shedding of cleaved S1 and conversion of S2 into its postfusion structure (S2*). Antigen sorting and trafficking may also induce the release of S protein-containing exosomes. The events shown will occur in the apical and/or basolateral surfaces of polarized (e.g., epithelial) cells. The Pfizer–BioNTech and Moderna constructs do not contain a mutated S1/S2 furin cleavage site. Further research will clarify the impact of the S1/S2 subunits stabilizing D614G (or other) mutation or of a mutated furin cleavage site in antigen distribution, the immunogenicity of the vaccine, and induced adverse events (AEs). Also shown are dendritic cells (professional antigen-presenting cells, APCs) engulfing circulating antigens, and antibody-mediated binding of B cells to cell-anchored antigens.