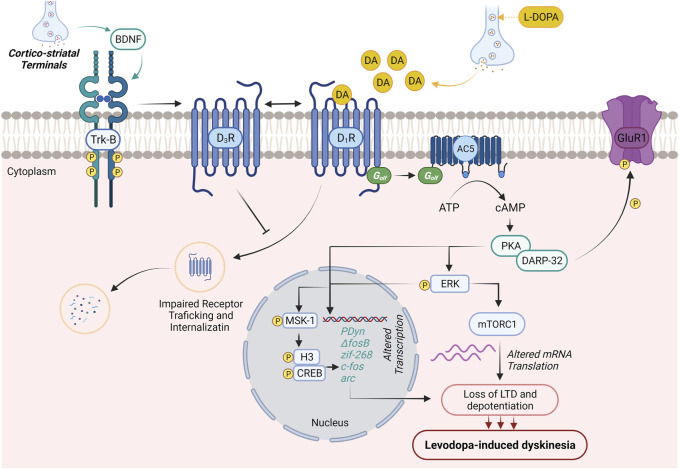
FIGURE 1.
Molecular neurobiology of levodopa-induced dyskinesia. DA production generally regulates voluntary movements. In PD patients, initial dopaminergic neurodegeneration in substantia nigra pars compacta is asymptomatic. Loss of striatal–nigral neuronal sensitizes the D1 receptor on the medium spiny neurons of the direct pathway. This results in initial motor symptoms of PD (dyskinesia). The treatment with L-DOPA improves the initial motor symptoms and promotes BDNF release from corticostriatal neurons. The expressed BDNF potentiates the expression of D3Rs through the activation of Trκ-B receptors in nigrostriatal medium spiny neurons. Enhanced expression of D3Rs suppresses internalization and abnormal trafficking of membrane-bound D1Rs, thus intensifying D1R sensitization and associated dyskinesia. Activation of D1R by DA (released through L-DOPA) results in the activation of D1R/Gαolf/adenylyl cyclase 5 (AC5) machinery in nigrostriatal medium spiny neurons and causes cAMP-mediated hyperexpression of protein kinase A (PKA) and DARPP-32. The abnormal PKA/DARPP-32 expression results in hyper-phosphorylation of GluR1 and promotes the excitability of medium spiny neurons contributing to the loss of long-term depression and depotentiation and development of LID. On the other hand, activation of D1R results in the Ras/Raf/MEK/ERK signaling pathway, which further potentiates Ras/Raf machinery and regulates various transcription and translation processes regulating LID. PKA/DARPP-32 and/or ERK/Elk/MSK1 signaling migrates to the nucleus, leads to phosphorylation of CREB/histone H3, and augments the expression of immediate early genes (prodynorphin and ΔFosB), which are reported to contribute to the development of LID. Activated ERK further elevates mTORC1 expression-mediated mRNA translation and worsens LID.