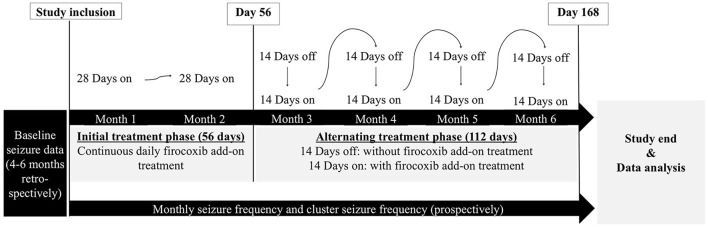
Figure 1.
Schematic illustration of the study design. The study course consisted of a continuous treatment period for the first 56 days, followed by a 14-day alternating treatment period for 112 days (total study period 168 days). The alternating treatment protocol was selected to lower the risk of potential firocoxib adverse events. Antiseizure medication was not changed during the firocoxib add-on phase, use of diazepam rectal tubes for emergency management of seizures was allowed. Clinical examinations and blood tests were performed on a regular base throughout the entire study period. Study participation was denied to any dog with pre-existing signs of gastrointestinal disease, renal disease or pre-treatment with any non-steroidal anti-inflammatory drug or glucocorticoids.