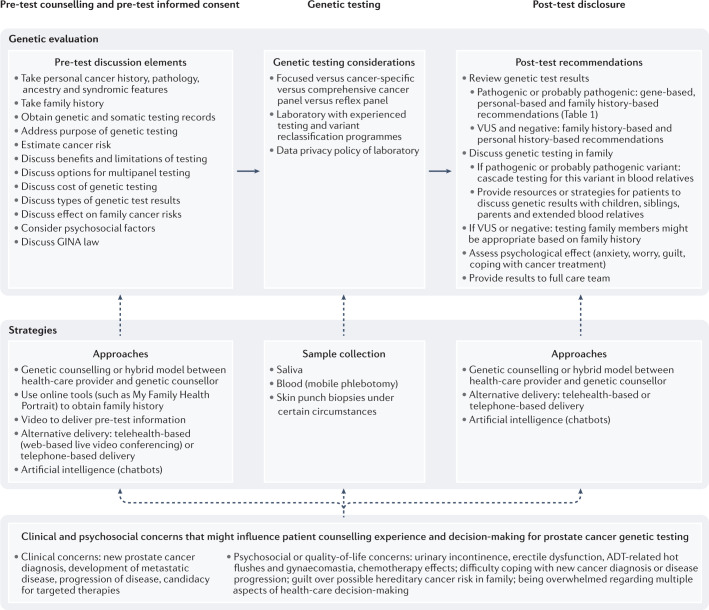
Fig. 1. Genetic evaluation process for patients with or at risk of developing prostate cancer.
The process and elements involved in pre-test counselling and informed consent, genetic testing and post-test disclosure. Special considerations in each step in the process and unique clinical and psychosocial concerns that can influence decision-making are shown. ADT, androgen-deprivation therapy; GINA, Genetic Information Nondiscrimination Act; VUS, variants of uncertain significance.