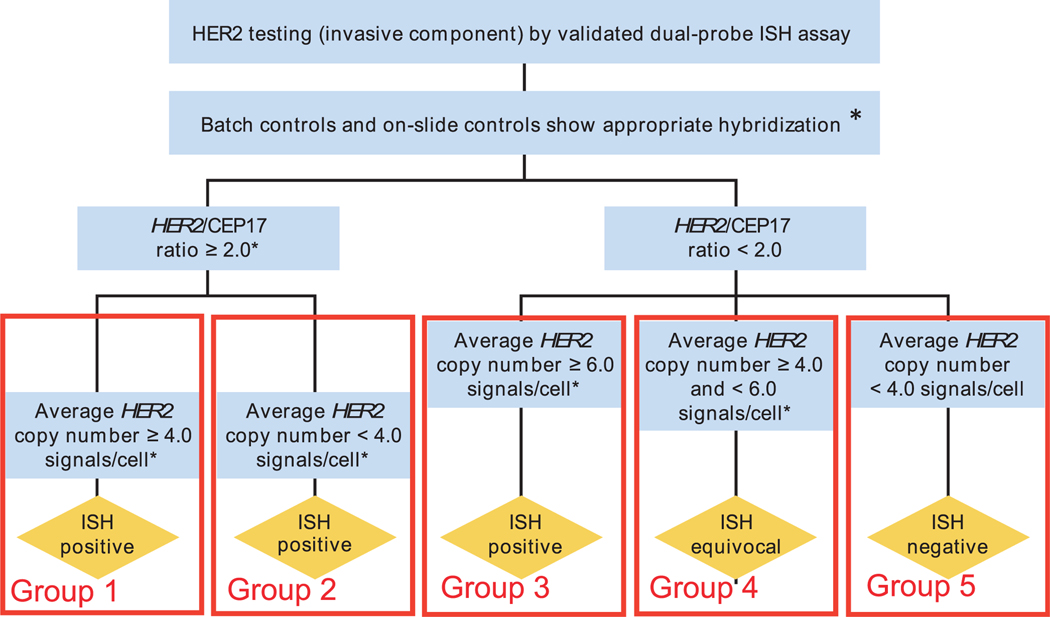
Figure 1.
Schematic diagram of the “Algorithm for evaluation of human epidermal growth factor receptor 2 (HER2) gene amplification by in situ hybridization (ISH) assay of the invasive component of a breast cancer specimen using a dual-signal (HER2 gene) assay (dual-probe ISH)” as published by the American Society of Clinical Oncology/College of American Pathologists (ASCO-CAP) guidelines committee,22 modified here by introduction of the groups 1 to 5 to identify the various ASCO-CAP fluorescence in situ hybridization (FISH) categories used in this analysis. Breast cancers with HER2:CEP17 ratios of 2.0 or greater are divided into two groups: one with an average HER2 gene copy number per tumor cell greater than/equal to 4.0 (ISH-positive, or our group 1) and one with an average HER2 gene copy number per tumor cell lower than 4.0 (ISH-positive, or our group 2). Breast cancers with HER2:CEP17 ratios lower than 2.0 are separated into three additional groups: one with an average HER2 gene copy number per tumor cell of 6.0 or greater (ISH-positive, or our group 3 [N or A]); another with an average HER2 gene copy number per tumor cell of 4.0 or greater but less than 6.0 (ISH-equivocal, or our group 4); and one with breast cancers containing an average HER2 gene copy number per tumor cell lower than 4.0 (ISH-negative, or our group 5). Therefore, according to the ASCO-CAP guidelines,22 breast cancers in groups 1, 2, and 3 are interpreted as “ISH positive,” group 4 as “ISH equivocal,” and group 5 as “ISH negative.” This figure has been modified from Figure 3 of the previously published article by Wolff et al,22 Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138(2):241–256, with permission from the Archives of Pathology & Laboratory Medicine. Copyright 2014 College of American Pathologists. (*) Observed in a homogeneous and contiguous population.