Abstract
Pancreatic ductal adenocarcinoma (PDAC) is an intractable cancer and a leading cause of cancer deaths worldwide. Over 90% of patients die within 1 year of diagnosis. Deaths from PDAC are increasing and it remains a cancer of substantial unmet need. A number of factors contribute to its poor prognosis: namely, late presentation, early metastases and limited systemic therapy options because of chemoresistance. A variety of research approaches underway are aimed at improving patient survival. Here, we review high-risk groups and efforts for early detection. We examine recent developments in the understanding of complex molecular and metabolic alterations which accompany PDAC. We explore artificial intelligence and biological targets for therapy and examine the role of tumour stroma and the immune microenvironment. We also review recent developments with respect to the PDAC microbiome. It is hoped that current research efforts will translate into earlier diagnosis, improvements in treatment and better outcomes for patients.
Keywords: Pancreatic ductal adenocarcinoma (PDAC), early detection, high-risk groups, artificial intelligence, tumour stroma, immune microenvironment, tumour microbiome
Introduction
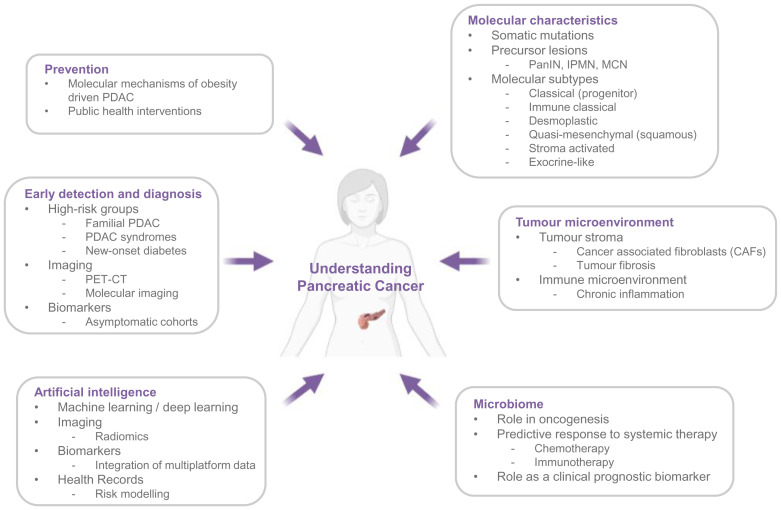
Pancreatic ductal adenocarcinoma (PDAC) has a 5-year survival of around 7 to 10%, which remains almost unchanged in 40 years1,2. Despite advances in uncovering the biology underpinning this disease, and improvements in diagnostic and cancer registration practices in some countries, deaths from PDAC are projected to increase in the coming years2. The only possibility of cure is appropriate surgery with adjuvant chemotherapy3. Patients classically present with obstructive jaundice (when the tumour arises in the pancreatic head); however, many present with vague symptoms if tumours arise in the body or tail of the pancreas4. Clinical vigilance is key. Thus, at presentation, almost 80 to 85% already have locally advanced or distant metastatic disease, and treatment options offering curative intent are limited5,6. Although patients can be brought to surgery using neo-adjuvant regimens7, those with metastatic disease are currently unsuitable for surgery. The success of systemic therapies has also been limited because of the intense resistance of the disease to current treatment regimens. Progress in treatment, including personalised approaches, in combination with earlier detection could significantly contribute to better patient outcome (Figure 1).
Figure 1. Research areas contributing to our understanding of pancreatic cancer.
IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm; PanIN, pancreatic intraepithelial neoplasia; PDAC, pancreatic ductal adenocarcinoma; PET-CT, positron emission tomography–computed tomography.
PDAC prevention
Risk factors for PDAC include a history of familial pancreatic cancer (FPC)8, hereditary syndromes predisposing to PDAC9, intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms10, chronic pancreatitis11, (especially when related to inherited homologues of human cationic trypsinogen [PRSS1]), obesity12, new-onset diabetes (NOD)13 and smoking14. The European Prospective Investigation into Cancer and Nutrition (EPIC) highlighted that individuals with high healthy lifestyle index scores (including measures of smoking, alcohol consumption, physical activity, adiposity and diet) have a decreased likelihood of developing PDAC15.
The rise in obesity and diseases associated with it, including type 2 diabetes, remains a major significant public health challenge in higher socio-economic index countries and increasingly in the developing world. Evidence from case-control and cohort studies suggests an association between obesity and PDAC16–18. Similarly, some animal and small case-control studies in humans have related fatty infiltration of the pancreas to PDAC development19. It has been suggested that obesity may drive tumorigenesis through the activation of KRAS signalling pathways, although our understanding of this process is in its infancy20. Understanding the molecular mechanisms behind obesity-driven tumorigenesis would facilitate the development of risk reduction strategies and targeted screening for obese individuals. Furthermore, understanding how to effectively implement public health interventions will be key in primary prevention at a population level.
Early detection and diagnosis of PDAC
Worldwide, there are currently no recommended population-wide screening programmes for PDAC in asymptomatic adults and this is due in part to the relatively low incidence of the disease in the general population (1.5–10 per 100,000 age-standardised rate, worldwide2). The World Health Organization advocates screening in all cancers, and anticipated increases in curative treatment are up to 30% from earlier detection (www.who.int/cancer/en/index.html). Five per cent to 10% of cases of PDAC are due to inherited risk factors: either a family history of the disease or germline mutations which give rise to PDAC syndromes9. The most studied of those syndromes include Peutz-Jeghers syndrome (STK11)21, hereditary breast-ovarian syndrome (BRCA2)22, familial atypical multiple mole melanoma (CDKN2A)23 and Lynch syndrome (MLH1/2/6)24. The International Cancer of the Pancreas Screening (CAPS) Consortium recommends screening for high-risk individuals, notably carriers of high-risk germline mutations, such as those mentioned above, or individuals with defined FPC. This screening should be offered in a research setting until the benefits, risks and costs of pancreatic cancer surveillance are elucidated25. Established research screening programmes include the following: the European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC), Liverpool, UK26; the North American National Familial Pancreatic Tumour Registry27 and the German National Case Collection for FPC28. There are specific guidelines for screening patients with chronic pancreatitis for the risk of pancreatic cancer. The current literature supports screening only for those patients with an autosomal dominant history of hereditary pancreatitis with or without a mutation in cationic trypsinogen (PRSS1 gene)29.
Individuals with NOD are the largest high-risk group who may benefit from screening30. The relationship between diabetes and PDAC is bidirectional31, and long-standing diabetes has a pooled relative risk of 2.1 for PDAC development, while individuals with NOD (duration of less than 1 year) have a 5.4-fold relative risk of developing PDAC32. For about 1% of individuals who are at least 50 years old and whose diabetes has been diagnosed, PDAC is the underlying cause of diabetes. PDAC-associated diabetes is a form of type 3c diabetes33. Hyperglycaemia in PDAC occurs up to 3 years before diagnosis and begins before pancreatic tumours are visible on imaging, suggesting that the pathophysiology of PDAC-associated glucose dysregulation is more than the destruction of the gland34. Further support for this is shown by the improvement in glucose dysregulation after surgical resection of the tumour35. Despite recent advances, our understanding of the biology underpinning the development of diabetes in PDAC is in its infancy. However, with about 80% of individuals presenting with glucose dysregulation at the time of diagnosis of PDAC, including a large proportion presenting with NOD, screening strategies for this high-risk group are urgently needed30. Cohorts of patients in both the USA (Chronic Pancreatitis, Diabetes and Pancreatic Cancer Consortium36) and the UK (UK Early Detection Initiative for Pancreatic Cancer37) have been established in response to this need to investigate how the presence of NOD may be used to expedite the detection of PDAC in this high-risk group.
The capacity for early detection is limited by current imaging modalities, which lack the sensitivity to identify small lesions. Endoscopic ultrasound (EUS), which has a sensitivity of 72% for identifying T1 and T2 cancers, is advocated in FPC but suffers from inter-user variability, inconsistency in patient access, and invasiveness38. Cross-sectional imaging is used for screening patients with hereditary pancreatitis, as the calcium load in these individuals will prevent adequate EUS assessment. Future developments with positron emission tomography (PET) and the use of molecular imaging techniques that target proteins overexpressed by the tumour, signalling pathways, or the stroma may improve detection of early lesions39,40. Further development of hyperpolarised magnetic resonance imaging, which can identify metabolic aberrations in the pancreas indicative of pre-neoplasia, may also prove to be a useful adjunct to early detection and screening41.
It is widely accepted that biomarkers will be essential in the refining of inclusion criteria for future PDAC screening programmes. Much research has been published in this area; however, no biomarkers have been carried through to clinical use. The failure to translate biomarker development into the clinic lies in part with the widespread validation of biomarkers using a diagnostic sample, which are compromised by late changes during tumorigenesis that are not seen in early-stage disease. Second, where existing pre-diagnostic cohorts exist, they often lack adequate control groups and do not contain data relevant to PDAC, such as diabetes status or presence of chronic pancreatitis, limiting the interpretation of findings. Finally, and perhaps most importantly, PDAC within the population is relatively rare, which demands high levels of specificity from biomarkers to minimise false-positive rates. To comprehensively test biomarker accuracy, large numbers of samples are required from target populations and these cohorts are hard to come by. Of those published biomarkers, carbohydrate antigen (CA) 19-9 remains the only validated biomarker clinically used in the management of PDAC. Although CA19-9 may hold future utility as a component in a panel of markers for the identification of PDAC, it lacks the sensitivity and specificity to act as a stand-alone biomarker for asymptomatic disease42–44. As our understanding of the molecular changes that occur during the transition from pre-neoplastic lesion to early PDAC and advanced disease improves, rational strategies have been applied to identify novel biomarkers across genomic, transcriptomic, metabolomic and proteomic platforms utilising a variety of specimens, including microvesicles (exosomes), ctDNA and methylated DNA45–47. New pre-diagnostic cohorts and large collaborative studies will be crucial to the development of biomarkers and their rapid translation to the clinic.
To meet with the demand for rapid evaluation of an increasing number of early-stage tumours, it is likely that diagnostic, prognostic and predictive algorithms, based on a combination of imaging, molecular and health data, will be needed. Here, artificial intelligence (AI), and more specifically machine learning (ML) and deep learning (DL) (a subset of ML applied to large datasets), have emerged as valuable tools for disease risk-stratification and identification in general populations. ML and DL automate analytical model building. They use a variety of operations to examine and compare small to large datasets to find common patterns and explore nuances. Both ML and DL can be performed in a supervised or unsupervised manner, with unsupervised learning training models and unlabelled data.
Early detection and management of pancreatic cancer rely on an understanding of the basic biology of the disease as well as an appreciation of the disease course at a population level (risk factors, disease progression, and treatment response). AI methods are making contributions here across the spectrum of basic research areas, and progress requires interdisciplinary collaboration between experts in AI, disease physiology and molecular cell biology. Perhaps most widely explored is the use of ML and DL approaches for the extraction and interpretation of features in medical imaging, although we are still only just scratching the surface of what is possible for image-based modelling of pancreatic cancer risk48. More recently, AI methods (ML and DL) have been explored to provide a better quantitative description of biological processes in PDAC49, to identify novel biomarkers50, and to undertake large-scale analysis of clinical records to develop algorithms for automatic identification of at-risk individuals51.
Increasingly extensive datasets are being generated as a result of advances in high-throughput proteomic, transcriptomic and genomic technologies. As ML researchers and practitioners gain more experience, the capacity for ML to interrogate and integrate this information to aid in our understanding of PDAC biology, and to inform how to manage and detect the disease at the earliest time point, will be immense. This will be an exciting area to follow in the coming years.
Targeting the molecular characteristics of PDAC
PDAC is preceded by the progression of precursor lesions, such as pancreatic intraepithelial neoplasia (PanIN), IPMN and mucinous cystic neoplasm. PanIN, which arises from a stepwise accumulation of somatic genetic mutations, is the most common of these premalignant lesions and represents a key target for early detection and treatment52. The major genetic mutations responsible for PanIN progression are well established. Grade 1 and 2 PanIN are characterised by a point mutation in the KRAS oncogene (90% of tumours)53, and telomerase shortening is also characteristic of this stage54. Grade 2 is associated with increased activation of CDKN2A and CDKN1A55. Grade 3 and 4 are associated with mutations in TP53 (50–70%) and SMAD4 (60–90%)56,57. There are many more somatic mutations described in PDAC specimens, and over 100 to 150 have been identified using next-generation sequencing58. Most somatic mutations occur in common pathways: RAS signalling, transforming growth factor beta (TGFβ) pathways, cell-cycle control, WNT signalling, NOTCH signalling and DNA damage repair59. As in other gastrointestinal tumours, the majority of somatic mutations are not currently targetable; however, there is some evidence that our understanding of underlying mutations is translating into clinical practice, including small molecule inhibitors of KRASG12C and PD-1 blockade in tumours deficient in the mismatch repair (MMR) genes MLH1 and MLH260,61.
Recent global transcriptomic analysis has allowed PDAC to be subclassified based on distinct molecular signatures. Early work classified these molecular subtypes as classical (progenitor), quasi-mesenchymal (squamous) and exocrine-like (aberrantly differentiated endocrine exocrine), although this classification was based on analysis of the epithelial aspects of PDAC tumours only and did not take into account the wider tumour microenvironment (TME)62. A more recent analysis of 309 resected PDAC tumours confirmed the progenitor, squamous and quasi-mesenchymal subsets but also further characterised the TME, identifying three additional subsets: desmoplastic, immune classical and stroma-activated63. It has been suggested that tumours of the squamous subset are associated with an adverse prognosis and are more resistant to current chemotherapy regimens compared with other subtypes; however, fully translating this work into clinical applications is still in the early stages64.
There are a number of research programmes aiming to apply the expansion of molecular subtypes of PDAC into clinical trials, and this is a major area of collaboration across industry and academia. The “Know Your Tumour Programme”, one such example, aims to determine whether targeting actionable molecular signatures can improve outcomes65,66. Much of this programme is focussed on patients with metastatic disease, and the most common actionable alterations are seen in DNA repair genes (BRCA1/2 or ATM mutations) or cell-cycle genes (CDK4/6 alterations). It should be noted that, despite genetic testing, the majority of patients (68%) enrolled in this trial nonetheless underwent standard-of-care chemotherapy with either FOLFIRINOX or gemcitabine with nab-paclitaxel rather than targeted therapy66. Other classes of drugs utilised based on molecular signatures of PDAC include inhibitors of MEK67 and PARP68. Data published from the “Know Your Tumour Programme” suggest that patients who underwent therapy that were matched to molecular profiling had better overall survival than those who did not (2.58 versus 1.51 years; P = 0.004), suggesting that it is feasible to tailor therapy on the basis of individual molecular characteristics. However, of the 1,856 patients who were referred to the programme, only 46 (2%) underwent matched therapy and this was due to the aggressiveness of the disease and limited treatments available at the time of the trial for some of the identified molecular signatures, such as NTRK alterations65,69. Additional limitations relating to targeted therapies should be noted. These include the difficulty in obtaining samples of adequate yield and quality for molecular subtyping in non-resected patients following fine-needle aspiration as well as intra-tumour heterogeneity of PDAC70. Targeted treatments based on molecular profiling, therefore, can currently be viewed as an encouraging proof-of-concept idea that requires ongoing global efforts before the majority of patients will benefit from such methods.
The role of tumour stroma in resistance to treatment
PDAC exhibits a strong desmoplastic reaction characterised by a hypoxic and hypovascular TME. This desmoplasia is argued to be a principal contributor of resistance to standard chemotherapy in PDAC71. Tissue stroma plays a role in the response to injury utilising the immune, vascular and connective tissue components within it. Understanding and trying to develop treatments to target aspects of the TME have therefore been the focus of research over the last decade. The characteristic dense stroma induced by PDAC includes an array of cell types, including cancer-associated fibroblasts (CAFs), inflammatory cells, blood vessels and nerve cells, as well as the extracellular matrix (ECM) produced by the CAFs, including collagen, fibronectin, laminin and hyaluronic acid72,73.
Pancreatic stellate cells (PSCs) are myofibroblast-like cells that are activated by PDAC cells and become CAFs, producing ECM resulting in fibrosis in the tumour74. This resultant desmoplasia creates a mechanical barrier around tumour cells and prevents vascularisation, which limits the delivery of chemotherapy and also immune cell infiltration75. Thus, a key area of research is to understand how to target the development of this dense stroma, either by targeting the extracellular components of the fibrosis itself or by targeting stromal cells (PSCs) and attempting to revert them to their quiescent form. Matrix metalloproteinases (MMPs) are a group of proteins that remodel the ECM. MMP276, MMP777,78 and MMP9 and tissue inhibitors of MMPs79 are shown in pre-clinical models to be differentially expressed between normal pancreas and PDAC, and higher levels are associated with worse prognosis and metastatic disease80. Marimastat81 and tanomastat82, two inhibitors of MMP, showed promising results in pre-clinical xenograft models of melanoma83, gastric cancer84 and colon cancer85. When these were applied to metastatic PDAC in phase 3 trials, however, there was no survival benefit81,82.
Interest in targeting hyaluronan within the ECM, stemming from the observation that high deposition of hyaluronan in PDAC is associated with poor prognosis, has developed over a number of years86. PEGPH20 is a human recombinant PH20 hyaluronidase, which in a mouse model led to depletion of hyaluronan, improved vascular permeability and increased drug delivery of gemcitabine and chemotherapeutic efficacy86. Clinical trials, however, have shown differing results; phase II trials of PEGPH20 with gemcitabine plus nab-paclitaxel have improved progression-free survival but reduced overall survival when combined with FOLFIRINOX87,88. Also, a subsequent phase III trial combining PEGPH20 with gemcitabine plus nab-paclitaxel did not improve overall survival compared with chemotherapy alone (hazard ratio = 1.00, P = 0.97)89. Much of this was due to the side effects of PEGPH20 plus the worsening of chemotoxic symptoms with FOLFIRINOX leading to reduced treatment regimens and dosages.
Subsequently, attempts to target signalling pathways responsible for the development of stroma rather than particular components of the ECM have focussed on the hedgehog signalling pathway. In utero, repression of endodermal Sonic hedgehog (SHH) by inhibin-βB and FGF2 allows the expression of Pdx1 and insulin, initiating pancreatic differentiation90. Dysregulated hedgehog signalling is implicated in pancreatic carcinogenesis91, and mouse models showed that hedgehog signalling promotes desmoplasia and antibody-mediated inhibition reduced this desmoplastic reaction92. There is also some evidence of paracrine stimulation of PSCs through this pathway93. Translation of this in phase II studies, however, has been disappointing. A trial combining the SHH inhibitor saridegib and gemcitabine in metastatic PDAC was stopped early in 2012 because it led to higher rates of progressive disease94. Similar trials combining gemcitabine with vismodegib also showed no benefit to overall or disease-free survival compared with chemotherapy alone95.
Efforts to target CAFs and PSCs in an attempt to reduce the desmoplastic reaction have focussed mainly on inhibition of fibroblast activation protein (FAP)96. Similar to results in colorectal cancer, the success of such treatments in PDAC has been limited96. The use of a small molecule inhibitor UAMC-1110 was not effective in a recent mouse model97. Indeed, studies of genetic deletion of fibroblasts in mouse models of PanIN and PDAC led to disease with more aggressive phenotypes, indicating that fibroblasts play a complex role in tumour development98. CAFs are also shown to be important for shaping the antitumour immune response99. Of particular importance is the role of CXCL12-CXCR4 signalling in stromal-immune crosstalk100. The COMBAT trial combined motixafortide, a CXCR4 inhibitor, with PD-1 inhibition in a phase IIa open-label study in metastatic PDAC and showed modest but insignificant changes to overall survival101. Thus, it is likely that future efforts will be focussed on exploiting reprogramming of the fibroblasts and the role they play in altering the TME rather than the cruder method of attempting to eliminate them. The results of ongoing studies focussing on CXCR4 inhibition (cemiplimab; ClinicalTrials.gov Identifier: NCT04177810) are eagerly awaited.
Understanding the immune microenvironment
The TME in PDAC is rich with immune cells, and it has long been established that chronic inflammation is an important characteristic of PDAC102. However, PDAC is a relatively immunologically “cold” tumour, and molecular profiling indicates that only a subset of the immune cells present within the tumour are immunologically active64,103. With respect to immune cells, the myeloid compartment dominates the TME and includes tumour-associated macrophages (TAMs), granulocytes and inflammatory monocytes. These are actively recruited to the TME during carcinogenesis orchestrated by KRAS mutations in the epithelial compartment104. TAMs can be categorised as either M1 or M2 macrophages; M1 generally shows tumoricidal activity acting via tumour necrosis factor (TNF), interleukin-12 (IL-12), IL-1α and interferon-gamma (INF-γ), and M2 produces anti-inflammatory tumour-promoting cytokines such as TGFβ and IL-10105. Myeloid-derived suppressor cells (MDSCs) are also produced from myeloid cells and these recruit regulatory T cells to the TME106,107. There is also evidence to suggest that this myeloid cell infiltration is critical for PDAC initiation (induction of immune checkpoint ligands) and it promotes the formation and maintenance of pre-neoplastic lesions108.
Immunotherapy is an active area of interest, most commonly aimed at augmenting the antitumour adaptive immune response. Most of the above-mentioned immunosuppressive cells, including TAM M2 macrophages109, TAM M1 macrophages110 and MDSCs111, have been targeted. Further targets include CD40 agonists112,113, chemokine modulation114 and immune checkpoint inhibitors115. Despite showing promise in other solid organ tumours, the most studied of these—CTLA-4 (ipilimumab), and PD-1 (nivolumab)—have been disappointing in PDAC116. Another approach is to enhance antigen presentation and drive the expansion of tumour-specific T-cell clones through “vaccination”. Whilst multiple studies have shown that it is possible to yield antigen-specific immunological responses in patients with PDAC, vaccination strategies alone might not be enough to generate clinically meaningful antitumour effects117.
The limitations of direct stroma or immune-based therapeutic targeting perhaps allude to the logic that a better strategy would be to exploit integrated aspects of the TME, such as specific points of biological convergence. An example of this is targeting cancer cell metabolism. Cancer cells maintain high glycolytic activity in order to grow as well as needing glutamine to fuel the tricarboxylic acid cycle (TCA) cycle118,119. It has been suggested that there is a symbiotic relationship between PDAC cells and the microenvironment, including CAFs and TAMs. CAFs release non-essential amino acids through enhanced autophagy to support tumour cell needs through the TCA cycle120. Moreover, there is some evidence that PDAC cells reprogramme the stroma into a tumour-promoting metabolic environment that hinders T cells121. Blocking glutamine metabolism augmented with anti-PD-1 led to cytotoxic T-cell activation and a reduction in hyaluronan synthesis in a mouse model of PDAC122,123. There is much to learn in this area, and limited data are available from the small number of trials conducted. However, these studies remain of great interest as they suggest that focussing on the metabolic remodelling of the TME may influence desmoplasia, cancer metabolism and the immune response in a more orchestrated way.
The role of the pancreas microbiome
Characterising the pancreatic tumour microbiome is providing insight into carcinogenesis. It is also uncovering the potential of the tumour microbiome as a therapeutic biomarker. Whilst the presence of bacteria in tumours is well recognised, their exact purpose or the consequences of their presence remain unclear. In an attempt to answer this question, Nejman et al. profiled the bacteria present in seven different human tumours, demonstrating that bacteria were located intracellularly in both cancer and immune cells124, raising the possibility that they influence the immune state of the tumour environment and have potential implications for responses to immunotherapy. Interestingly, the bacterial composition of tumours varied between tumour types.
The notion that certain microbes play a causative role in oncogenesis is gaining momentum125,126. Significant variation in methodology and results, however, currently prevents consensus opinion. Elevated levels of intracystic bacterial DNA were found in patients with IPMN (both with high-grade dysplasia and with cancer)127, raising pertinent questions regarding the potential of iatrogenic bacterial translocation via endoscopy. Of course, it must be appreciated that other routes of translocation exist. Furthermore, exploration of pancreatic cystic fluid (PCF) has revealed the existence of a unique bacterial ecosystem, which may play a role in oncogenesis128.
The pancreatic cancer microbiome may also act as a clinical prognostic biomarker. Via 16S rRNA gene sequencing, the tumour microbiome of long-term survivors (LTSs) was compared with that of short-term survivors (STSs)129. LTSs were found to have a more diverse tumour microbiome, and an intra-tumoral microbiome signature was found to be predictive of long-term survival. Human-to-mice faecal microbial transplants demonstrated attenuated tumour growth and immune cell infiltration with LTS faeces when compared with STS faeces. This pivotal study demonstrated that crosstalk occurs between the gut and tumour microbiome, which can directly influence and predict the outcome of disease129.
The immunosuppressive environment of PDAC to date has hindered the effective use of immunotherapy. Targeting the intra-tumoural microbiome may be a strategy to increase efficacy. Bacterial ablation of tumours results in immunogenic reprogramming of the PDAC microenvironment, reducing MDSCs, increasing macrophage differentiation, and promoting CD4+ T helper cells and CD8+ T-cell activation130. Reducing the bacterial content of tumours upregulates PD-1 expression, increasing the efficacy of checkpoint-targeted immunotherapy130. A number of studies in tumours such as lung and melanoma have addressed the proposal that gut dysbiosis may affect response to treatments131–133. The idea that we may be able to use microbiota composition to define groups most likely to respond to treatment is gaining traction and may help inform future clinical trials.
It can no longer be assumed that the bacterial populations found inside and around PDAC are merely environmental bystanders. What remains unclear is whether bacteria in these regions contribute to causing the cancer or whether they populate as a result of oncogenesis. What is recognised, however, is that crosstalk between organs and their microbiomes exists and is far greater than anticipated. A barrier to translating scientific findings into large-scale clinical trials and clinically meaningful results is the issue of bias in the studies published, which can occur at any stage of the analysis. Consequently, there are relatively few clinical trials aimed at evaluating therapies manipulating the pancreatic microbiome134. The results from emerging clinical trials in this area are thus eagerly awaited.
Conclusions
PDAC remains a major global health problem. Improvements in survival have been made over the last 20 years because of advances in perioperative care, meaning that patients who undergo surgery can also benefit from adjuvant therapy. Many patients continue to present late with a disease that is too advanced for surgical intervention with curative intent, and this remains a significant challenge. Our understanding of high-risk groups and how to detect disease early in these groups continues to be central to improving outcomes, and ongoing research using high-risk cohorts is vitally important. Developments in AI and ML will hopefully improve early detection initiatives taking into account large datasets. There has been an increase in our understanding of the biology of PDAC, its microenvironment and the microbiome across the genetic, epigenetic, transcriptomic, proteomic and metabolomic spectrums. The translation of these findings into clinical trials remains an aim for the future.
Acknowledgements
Figure 1 was created with BioRender.com.
The peer reviewers who approve this article are:
Ashok K Saluja, Departments of Surgery, Miller School of Medicine, University of Miami, Miami, USA
Shweta Lavania, Departments of Surgery, Miller School of Medicine, University of Miami, Miami, USA
Si Young Song, Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea
Funding Statement
The authors declare that no grants were involved in supporting this work.
References
- 1. Arnold M, Rutherford MJ, Bardot A, et al. : Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019; 20(11): 1493–505. 10.1016/S1470-2045(19)30456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. : Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71(3): 209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 3. Springfeld C, Jäger D, Büchler MW, et al. : Chemotherapy for pancreatic cancer. Presse Med. 2019; 48(3 Pt 2): e159–e174. 10.1016/j.lpm.2019.02.025 [DOI] [PubMed] [Google Scholar]
- 4. Walter FM, Mills K, Mendonça SC, et al. : Symptoms and patient factors associated with diagnostic intervals for pancreatic cancer (SYMPTOM pancreatic study): A prospective cohort study. Lancet Gastroenterol Hepatol. 2016; 1(4): 298–306. 10.1016/S2468-1253(16)30079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Y, Abel GA, Hamilton W, et al. : Diagnosis of cancer as an emergency: A critical review of current evidence. Nat Rev Clin Oncol. 2017; 14(1): 45–56. 10.1038/nrclinonc.2016.155 [DOI] [PubMed] [Google Scholar]
- 6. Stapley S, Peters TJ, Neal RD, et al. : The risk of pancreatic cancer in symptomatic patients in primary care: A large case-control study using electronic records. Br J Cancer. 2012; 106(12): 1940–4. 10.1038/bjc.2012.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy JE, Wo JY, Ryan DP, et al. : Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018; 4(7): 963–9. 10.1001/jamaoncol.2018.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez E, La Vecchia C, D'Avanzo B, et al. : Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 1994; 3(3): 209–12. [PubMed] [Google Scholar]
- 9. Solomon S, Das S, Brand R, et al. : Inherited pancreatic cancer syndromes. Cancer J. 2012; 18(6): 485–91. 10.1097/PPO.0b013e318278c4a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vege SS, Ziring B, Jain R, et al. : American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015; 148(4): 819–22; quize12-3. 10.1053/j.gastro.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 11. Kirkegård J, Mortensen FV, Cronin-Fenton D: Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017; 112(9): 1366–72. 10.1038/ajg.2017.218 [DOI] [PubMed] [Google Scholar]
- 12. Stolzenberg-Solomon RZ, Schairer C, Moore S, et al. : Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2013; 98(4): 1057–65. 10.3945/ajcn.113.058123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen DK, Korc M, Petersen GM, et al. : Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes. 2017; 66(5): 1103–10. 10.2337/db16-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Molina-Montes E, van Hoogstraten L, Gomez-Rubio P, et al. : Pancreatic Cancer Risk in Relation to Lifetime Smoking Patterns, Tobacco Type, and Dose-Response Relationships. Cancer Epidemiol Biomarkers Prev. 2020; 29(5): 1009–18. 10.1158/1055-9965.EPI-19-1027 [DOI] [PubMed] [Google Scholar]
- 15. Naudin S, Viallon V, Hashim D, et al. : Healthy lifestyle and the risk of pancreatic cancer in the EPIC study. Eur J Epidemiol. 2020; 35(10): 975–86. 10.1007/s10654-019-00559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 16. Calle EE, Rodriguez C, Walker-Thurmond K, et al. : Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003; 348(17): 1625–38. 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 17. Berrington de Gonzalez A, Sweetland S, Spencer E: A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer. 2003; 89(3): 519–23. 10.1038/sj.bjc.6601140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pothuraju R, Rachagani S, Junker WM, et al. : Pancreatic cancer associated with obesity and diabetes: An alternative approach for its targeting. J Exp Clin Cancer Res. 2018; 37(1): 319. 10.1186/s13046-018-0963-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takahashi M, Hori M, Ishigamori R, et al. : Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018; 109(10): 3013–23. 10.1111/cas.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eibl G, Rozengurt E: KRAS, YAP, and obesity in pancreatic cancer: A signaling network with multiple loops. Semin Cancer Biol. 2019; 54: 50–62. 10.1016/j.semcancer.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benzel J, Fendrich V: Familial Pancreatic Cancer. Oncol Res Treat. 2018; 41(10): 611–8. 10.1159/000493473 [DOI] [PubMed] [Google Scholar]
- 22. Brose MS, Rebbeck TR, Calzone KA, et al. : Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002; 94(18): 1365–72. 10.1093/jnci/94.18.1365 [DOI] [PubMed] [Google Scholar]
- 23. Vasen HF, Gruis NA, Frants RR, et al. : Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer. 2000; 87(6): 809–11. [PubMed] [Google Scholar]
- 24. Kastrinos F, Mukherjee B, Tayob N, et al. : Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009; 302(16): 1790–5. 10.1001/jama.2009.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goggins M, Overbeek KA, Brand R, et al. : Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020; 69(1): 7–17. 10.1136/gutjnl-2019-319352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grocock CJ, Vitone LJ, Harcus MJ, et al. : Familial pancreatic cancer: A review and latest advances. Adv Med Sci. 2007; 52: 37–49. [PubMed] [Google Scholar]
- 27. Klein AP, Hruban RH, Brune KA, et al. : Familial pancreatic cancer. Cancer J. 2001; 7(4): 266–73. [PubMed] [Google Scholar]
- 28. Schneider R, Slater EP, Sina M, et al. : German national case collection for familial pancreatic cancer (FaPaCa): Ten years experience. Fam Cancer. 2011; 10(2): 323–30. 10.1007/s10689-010-9414-x [DOI] [PubMed] [Google Scholar]
- 29. Greenhalf W, Lévy P, Gress T, et al. : International consensus guidelines on surveillance for pancreatic cancer in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology. 2020; 20(5): 910–8. 10.1016/j.pan.2020.05.011 [DOI] [PubMed] [Google Scholar]
- 30. Pereira SP, Oldfield L, Ney A, et al. : Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020; 5(7): 698–710. 10.1016/S2468-1253(19)30416-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma A, Chari ST: Pancreatic Cancer and Diabetes Mellitus. Curr Treat Options Gastroenterol. 2018; 16(4): 466–78. 10.1007/s11938-018-0197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ben Q, Xu M, Ning X, et al. : Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer. 2011; 47(13): 1928–37. 10.1016/j.ejca.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 33. Singhi AD, Koay EJ, Chari ST, et al. : Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology. 2019; 156(7): 2024–40. 10.1053/j.gastro.2019.01.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma A, Smyrk TC, Levy MJ, et al. : Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology. 2018; 155(2): 490–500.e2. 10.1053/j.gastro.2018.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chari ST, Zapiach M, Yadav D, et al. : Beta-cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology. 2005; 5(2–3): 229–33. 10.1159/000085276 [DOI] [PubMed] [Google Scholar]
- 36. Serrano J, Andersen DK, Forsmark CE, et al. : Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer: From Concept to Reality. Pancreas. 2018; 47(10): 1208–12. 10.1097/MPA.0000000000001167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oldfield L, Hanson R, Greenhalf W, et al. : UK Early Detection Initiative (UK-EDI) for Pancreatic Cancer. Pancreatology. 2020; 20(Supplement 1): S120–S121. 10.1016/j.pan.2020.07.218 [DOI] [Google Scholar]
- 38. Li JH, He R, Li YM, et al. : Endoscopic ultrasonography for tumor node staging and vascular invasion in pancreatic cancer: A meta-analysis. Dig Surg. 2014; 31(4–5): 297–305. 10.1159/000368089 [DOI] [PubMed] [Google Scholar]
- 39. England CG, Hernandez R, Eddine SB, et al. : Molecular Imaging of Pancreatic Cancer with Antibodies. Mol Pharm. 2016; 13(1): 8–24. 10.1021/acs.molpharmaceut.5b00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alauddin MM, de Palatis L: Current and Future Trends in Early Detection of Pancreatic Cancer: Molecular Targets and PET Probes. Curr Med Chem. 2015; 22(29): 3370–89. 10.2174/0929867322666150821094015 [DOI] [PubMed] [Google Scholar]
- 41. Serrao EM, Kettunen MI, Rodrigues TB, et al. : MRI with hyperpolarised [1-13C]pyruvate detects advanced pancreatic preneoplasia prior to invasive disease in a mouse model. Gut. 2016; 65(3): 465–75. 10.1136/gutjnl-2015-310114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindgaard SC, Sztupinszki Z, Maag E, et al. : Circulating Protein Biomarkers for Use in Pancreatic Ductal Adenocarcinoma Identification. Clin Cancer Res. 2021; 27(9): 2592–603. 10.1158/1078-0432.CCR-20-4215 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 43. Fahrmann JF, Schmidt CM, Mao X, et al. : Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology. 2021; 160(4): 1373–1383.e6. 10.1053/j.gastro.2020.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 44. Root A, Allen P, Tempst P, et al. : Protein Biomarkers for Early Detection of Pancreatic Ductal Adenocarcinoma: Progress and Challenges. Cancers (Basel). 2018; 10(3): 67. 10.3390/cancers10030067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagpal SJS, Bamlet WR, Kudva YC, et al. : Comparison of Fasting Human Pancreatic Polypeptide Levels Among Patients With Pancreatic Ductal Adenocarcinoma, Chronic Pancreatitis, and Type 2 Diabetes Mellitus. Pancreas. 2018; 47(6): 738–41. 10.1097/MPA.0000000000001077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim J, Bamlet WR, Oberg AL, et al. : Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci Transl Med. 2017; 9(398): eaah5583. 10.1126/scitranslmed.aah5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoshino A, Kim HS, Bojmar L, et al. : Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell. 2020; 182(4): 1044–1061.e18. 10.1016/j.cell.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 48. Kenner B, Chari ST, Kelsen D, et al. : Artificial Intelligence and Early Detection of Pancreatic Cancer: 2020 Summative Review. Pancreas. 2021; 50(3): 251–79. 10.1097/MPA.0000000000001762 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 49. Sinkala M, Mulder N, Martin D: Machine Learning and Network Analyses Reveal Disease Subtypes of Pancreatic Cancer and their Molecular Characteristics. Sci Rep. 2020; 10(1): 1212. 10.1038/s41598-020-58290-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 50. Long NP, Jung KH, Anh NH, et al. : An Integrative Data Mining and Omics-Based Translational Model for the Identification and Validation of Oncogenic Biomarkers of Pancreatic Cancer. Cancers (Basel). 2019; 11(2): 155. 10.3390/cancers11020155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen Q, Cherry DR, Nalawade V, et al. : Clinical Data Prediction Model to Identify Patients With Early-Stage Pancreatic Cancer. JCO Clin Cancer Inform. 2021; 5: 279–87. 10.1200/CCI.20.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 52. Fischer CG, Wood LD: From somatic mutation to early detection: Insights from molecular characterization of pancreatic cancer precursor lesions. J Pathol. 2018; 246(4): 395–404. 10.1002/path.5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mizrahi JD, Surana R, Valle JW, et al. : Pancreatic cancer. Lancet. 2020; 395(10242): 2008–20. 10.1016/S0140-6736(20)30974-0 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 54. van Heek NT, Meeker AK, Kern SE, et al. : Telomere Shortening Is Nearly Universal in Pancreatic Intraepithelial Neoplasia. Am J Pathol. 2002; 161(5): 1541–7. 10.1016/S0002-9440(10)64432-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kanda M, Matthaei H, Wu J, et al. : Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012; 142(4): 730–733.e9. 10.1053/j.gastro.2011.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo J, Xie K, Zheng S: Molecular Biomarkers of Pancreatic Intraepithelial Neoplasia and Their Implications in Early Diagnosis and Therapeutic Intervention of Pancreatic Cancer. Int J Biol Sci. 2016; 12(3): 292–301. 10.7150/ijbs.14995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yachida S, Iacobuzio-Donahue CA: The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009; 133(3): 413–22. 10.5858/133.3.413 [DOI] [PubMed] [Google Scholar]
- 58. Martincorena I, Campbell PJ: Somatic mutation in cancer and normal cells. Science. 2015; 349(6255): 1483–9. 10.1126/science.aab4082 [DOI] [PubMed] [Google Scholar]
- 59. Ying H, Dey P, Yao W, et al. : Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016; 30(4): 355–85. 10.1101/gad.275776.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Canon J, Rex K, Saiki AY, et al. : The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019; 575(7781): 217–23. 10.1038/s41586-019-1694-1 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 61. Le DT, Durham JN, Smith KN, et al. : Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017; 357(6349): 409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 62. Collisson EA, Sadanandam A, Olson P, et al. : Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011; 17(4): 500–3. 10.1038/nm.2344 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 63. Puleo F, Nicolle R, Blum Y, et al. : Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology. 2018; 155(6): 1999–2013.e3. 10.1053/j.gastro.2018.08.033 [DOI] [PubMed] [Google Scholar]
- 64. Bailey P, Chang DK, Nones K, et al. : Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016; 531(7592): 47–52. 10.1038/nature16965 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 65. Pishvaian MJ, Bender RJ, Halverson D, et al. : Molecular Profiling of Patients with Pancreatic Cancer: Initial Results from the Know Your Tumor Initiative. Clin Cancer Res. 2018; 24(20): 5018–27. 10.1158/1078-0432.CCR-18-0531 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 66. Pishvaian MJ, Blais EM, Brody JR, et al. : Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020; 21(4): 508–18. 10.1016/S1470-2045(20)30074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 67. Guan M, Bender RJ, Pishvaian MJ, et al. : Molecular and clinical characterization of BRAF mutations in pancreatic ductal adenocarcinomas (PDACs). JCO. 2018; 36(4): 214. 10.1200/JCO.2018.36.4_suppl.214 [DOI] [Google Scholar]
- 68. Golan T, Hammel P, Reni M, et al. : Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019; 381(4): 317–27. 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 69. Laeseke PF, Chen R, Jeffrey RB, et al. : Combining in Vitro Diagnostics with in Vivo Imaging for Earlier Detection of Pancreatic Ductal Adenocarcinoma: Challenges and Solutions. Radiology. 2015; 277(3): 644–61. 10.1148/radiol.2015141020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Asokkumar R, Yung Ka C, Loh T, et al. : Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): A randomized study. Endosc Int Open. 2019; 7(8): E955–E963. 10.1055/a-0903-2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Whatcott CJ, Diep CH, Jiang P, et al. : Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015; 21(15): 3561–8. 10.1158/1078-0432.CCR-14-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kleeff J, Beckhove P, Esposito I, et al. : Pancreatic cancer microenvironment. Int J Cancer. 2007; 121(4): 699–705. 10.1002/ijc.22871 [DOI] [PubMed] [Google Scholar]
- 73. Neoptolemos JP, Kleeff J, Michl P, et al. : Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018; 15(6): 333–48. 10.1038/s41575-018-0005-x [DOI] [PubMed] [Google Scholar]
- 74. Barrera LN, Evans A, Lane B, et al. : Fibroblasts from Distinct Pancreatic Pathologies Exhibit Disease-Specific Properties. Cancer Res. 2020; 80(13): 2861–73. 10.1158/0008-5472.CAN-19-3534 [DOI] [PubMed] [Google Scholar]
- 75. Provenzano PP, Cuevas C, Chang AE, et al. : Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012; 21(3): 418–29. 10.1016/j.ccr.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 76. Okada Y, Eibl G, Guha S, et al. : Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004; 21(4): 285–92. 10.1023/b:clin.0000046131.24625.54 [DOI] [PubMed] [Google Scholar]
- 77. Crawford HC, Scoggins CR, Washington MK, et al. : Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002; 109(11): 1437–44. 10.1172/JCI15051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fukuda A, Wang SC, Morris JP, et al. : Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011; 19(4): 441–55. 10.1016/j.ccr.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tang Y, Kesavan P, Nakada MT, et al. : Tumor-stroma interaction: Positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res. 2004; 2(2): 73–80. [PubMed] [Google Scholar]
- 80. Jones LE, Humphreys MJ, Campbell F, et al. : Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: Increased expression of matrix metalloproteinase-7 predicts poor survival. Clin Cancer Res. 2004; 10(8): 2832–45. 10.1158/1078-0432.ccr-1157-03 [DOI] [PubMed] [Google Scholar]
- 81. Bramhall SR, Schulz J, Nemunaitis J, et al. : A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002; 87(2): 161–7. 10.1038/sj.bjc.6600446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moore MJ, Hamm J, Dancey J, et al. : Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003; 21(17): 3296–302. 10.1200/JCO.2003.02.098 [DOI] [PubMed] [Google Scholar]
- 83. Napoli S, Scuderi C, Gattuso G, et al. : Functional Roles of Matrix Metalloproteinases and Their Inhibitors in Melanoma. Cells. 2020; 9(5): 1151. 10.3390/cells9051151 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 84. Kimata M, Otani Y, Kubota T, et al. : Matrix metalloproteinase inhibitor, marimastat, decreases peritoneal spread of gastric carcinoma in nude mice. Jpn J Cancer Res. 2002; 93(7): 834–41. 10.1111/j.1349-7006.2002.tb01326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cong J, Gong J, Yang C, et al. : miR-22 Suppresses Tumor Invasion and Metastasis in Colorectal Cancer by Targeting NLRP3. Cancer Manag Res. 2020; 12: 5419–29. 10.2147/CMAR.S255125 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 86. Jacobetz MA, Chan DS, Neesse A, et al. : Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013; 62(1): 112–20. 10.1136/gutjnl-2012-302529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hingorani SR, Zheng L, Bullock AJ, et al. : HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018; 36(4): 359–66. 10.1200/JCO.2017.74.9564 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 88. Ramanathan RK, McDonough SL, Philip PA, et al. : Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J Clin Oncol. 2019; 37(13): 1062–9. 10.1200/JCO.18.01295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van Cutsem E, Tempero MA, Sigal D, et al. : Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J Clin Oncol. 2020; 38(27): 3185–94. 10.1200/JCO.20.00590 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 90. Kawahira H, Ma NH, Tzanakakis ES, et al. : Combined activities of hedgehog signaling inhibitors regulate pancreas development. Development. 2003; 130(20): 4871–9. 10.1242/dev.00653 [DOI] [PubMed] [Google Scholar]
- 91. Haque I, De A, Majumder M, et al. : The matricellular protein CCN1/Cyr61 is a critical regulator of Sonic Hedgehog in pancreatic carcinogenesis. J Biol Chem. 2012; 287(46): 38569–79. 10.1074/jbc.M112.389064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Steele NG, Biffi G, Kemp SB, et al. : Inhibition of Hedgehog Signaling Alters Fibroblast Composition in Pancreatic Cancer. Clin Cancer Res. 2021; 27(7): 2023–37. 10.1158/1078-0432.CCR-20-3715 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 93. Han L, Ma J, Duan W, et al. : Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway. Oncotarget. 2016; 7(14): 18146–58. 10.18632/oncotarget.7776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ko AH, LoConte N, Tempero MA, et al. : A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas. 2016; 45(3): 370–5. 10.1097/MPA.0000000000000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Catenacci DVT, Junttila MR, Karrison T, et al. : Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015; 33(36): 4284–92. 10.1200/JCO.2015.62.8719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hofheinz RD, Al-Batran SE, Hartmann F, et al. : Stromal antigen targeting by a humanised monoclonal antibody: An early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003; 26(1): 44–8. 10.1159/000069863 [DOI] [PubMed] [Google Scholar]
- 97. Gunderson AJ, Yamazaki T, McCarty K, et al. : Blockade of fibroblast activation protein in combination with radiation treatment in murine models of pancreatic adenocarcinoma. PLoS One. 2019; 14(2): e0211117. 10.1371/journal.pone.0211117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. : Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014; 25(6): 719–34. 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 99. Elyada E, Bolisetty M, Laise P, et al. : Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019; 9(8): 1102–23. 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 100. Blair AB, Kim VM, Muth ST, et al. : Dissecting the Stromal Signaling and Regulation of Myeloid Cells and Memory Effector T Cells in Pancreatic Cancer. Clin Cancer Res. 2019; 25(17): 5351–63. 10.1158/1078-0432.CCR-18-4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bockorny B, Semenisty V, Macarulla T, et al. : BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat Med. 2020; 26(6): 878–85. 10.1038/s41591-020-0880-x [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 102. Yadav D, Lowenfels AB: The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013; 144(6): 1252–61. 10.1053/j.gastro.2013.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Danilova L, Ho WJ, Zhu Q, et al. : Programmed Cell Death Ligand-1 (PD-L1) and CD8 Expression Profiling Identify an Immunologic Subtype of Pancreatic Ductal Adenocarcinomas with Favorable Survival. Cancer Immunol Res. 2019; 7(6): 886–95. 10.1158/2326-6066.CIR-18-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dougan SK: The Pancreatic Cancer Microenvironment. Cancer J. 2017; 23(6): 321–5. 10.1097/PPO.0000000000000288 [DOI] [PubMed] [Google Scholar]
- 105. Ruffell B, Affara NI, Coussens LM: Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012; 33(3): 119–26. 10.1016/j.it.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ostrand-Rosenberg S, Sinha P: Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009; 182(8): 4499–506. 10.4049/jimmunol.0802740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gabrilovich DI: Myeloid-Derived Suppressor Cells. Cancer Immunol Res. 2017; 5(1): 3–8. 10.1158/2326-6066.CIR-16-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang Y, Yan W, Mathew E, et al. : Epithelial-Myeloid cell crosstalk regulates acinar cell plasticity and pancreatic remodeling in mice. eLife. 2017; 6: e27388. 10.7554/eLife.27388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cannarile MA, Weisser M, Jacob W, et al. : Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017; 5(1): 53. 10.1186/s40425-017-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Panni RZ, Herndon JM, Zuo C, et al. : Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med. 2019; 11(499): 10.1126/scitranslmed.aau9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Steele CW, Karim SA, Leach JDG, et al. : CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2016; 29(6): 832–45. 10.1016/j.ccell.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 112. Beatty GL, Chiorean EG, Fishman MP, et al. : CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011; 331(6024): 1612–6. 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 113. Vonderheide RH: CD40 Agonist Antibodies in Cancer Immunotherapy. Annu Rev Med. 2020; 71: 47–58. 10.1146/annurev-med-062518-045435 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 114. Noel M, O'Reilly EM, Wolpin BM, et al. : Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest New Drugs. 2020; 38(3): 800–11. 10.1007/s10637-019-00830-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 115. Silva IP, Long GV: Systemic therapy in advanced melanoma: Integrating targeted therapy and immunotherapy into clinical practice. Curr Opin Oncol. 2017; 29(6): 484–92. 10.1097/CCO.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 116. Tan E, El-Rayes B: Pancreatic Cancer and Immunotherapy: Resistance Mechanisms and Proposed Solutions. J Gastrointest Cancer. 2019; 50(1): 1–8. 10.1007/s12029-018-0179-z [DOI] [PubMed] [Google Scholar]
- 117. Salman B, Zhou D, Jaffee EM, et al. : Vaccine therapy for pancreatic cancer. Oncoimmunology. 2013; 2(12): e26662. 10.4161/onci.26662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Altman BJ, Stine ZE, Dang CV: From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016; 16(10): 619–34. 10.1038/nrc.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Warburg O: On the origin of cancer cells. Science. 1956; 123(3191): 309–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 120. Zhao H, Yang L, Baddour J, et al. : Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife. 2016; 5: e10250. 10.7554/eLife.10250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ho WJ, Jaffee EM, Zheng L: The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020; 17(9): 527–40. 10.1038/s41571-020-0363-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 122. Leone RD, Zhao L, Englert JM, et al. : Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019; 366(6468): 1013–21. 10.1126/science.aav2588 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 123. Sharma NS, Gupta VK, Garrido VT, et al. : Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J Clin Invest. 2020; 130(1): 451–65. 10.1172/JCI127515 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 124. Nejman D, Livyatan I, Fuks G, et al. : The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020; 368(6494): 973–80. 10.1126/science.aay9189 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 125. Fan X, Alekseyenko AV, Wu J, et al. : Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut. 2018; 67(1): 120–7. 10.1136/gutjnl-2016-312580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vogtmann E, Han Y, Caporaso JG, et al. : Oral microbial community composition is associated with pancreatic cancer: A case-control study in Iran. Cancer Med. 2020; 9(2): 797–806. 10.1002/cam4.2660 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 127. Gaiser RA, Halimi A, Alkharaan H, et al. : Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut. 2019; 68(12): 2186–94. 10.1136/gutjnl-2018-317458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li S, Fuhler GM, Bn N, et al. : Pancreatic cyst fluid harbors a unique microbiome. Microbiome. 2017; 5(1): 147. 10.1186/s40168-017-0363-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Riquelme E, Zhang Y, Zhang L, et al. : Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019; 178(4): 795–806.e12. 10.1016/j.cell.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 130. Pushalkar S, Hundeyin M, Daley D, et al. : The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018; 8(4): 403–16. 10.1158/2159-8290.CD-17-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 131. Routy B, Le Chatelier E, Derosa L, et al. : Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018; 359(6371): 91–7. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 132. Gopalakrishnan V, Spencer CN, Nezi L, et al. : Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018; 359(6371): 97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Matson V, Fessler J, Bao R, et al. : The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018; 359(6371): 104–8. 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Katayama ES, Hue JJ, Bajor DL, et al. : A comprehensive analysis of clinical trials in pancreatic cancer: What is coming down the pike? Oncotarget. 2020; 11(38): 3489–501. 10.18632/oncotarget.27727 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation