Summary
Background
In COVACTA, a randomised, placebo-controlled trial in patients hospitalised with coronavirus disease-19 (COVID-19), tocilizumab did not improve 28-day mortality, but shortened hospital and intensive care unit stay. Longer-term effects of tocilizumab in patients with COVID-19 are unknown. Therefore, the efficacy and safety of tocilizumab in COVID-19 beyond day 28 and its impact on Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) clearance and antibody response in COVACTA were investigated.
Methods
Adults in Europe and North America hospitalised with COVID-19 (N = 452) between April 3, 2020 and May 28, 2020 were randomly assigned (2:1) to double-blind intravenous tocilizumab or placebo and assessed for efficacy and safety through day 60. Assessments included mortality, time to hospital discharge, SARS-CoV-2 viral load in nasopharyngeal swab and serum samples, and neutralising anti-SARS-CoV-2 antibodies in serum. ClinicalTrials.gov registration: NCT04320615.
Findings
By day 60, 24·5% (72/294) of patients in the tocilizumab arm and 25·0% (36/144) in the placebo arm died (weighted difference –0·5% [95% CI –9·1 to 8·0]), and 67·0% (197/294) in the tocilizumab arm and 63·9% (92/144) in the placebo arm were discharged from the hospital. Serious infections occurred in 24·1% (71/295) of patients in the tocilizumab arm and 29·4% (42/143) in the placebo arm. Median time to negative reverse transcriptase–quantitative polymerase chain reaction result in nasopharyngeal/oropharyngeal samples was 15·0 days (95% CI 14·0 to 21·0) in the tocilizumab arm and 21·0 days (95% CI 14·0 to 28·0) in the placebo arm. All tested patients had positive test results for neutralising anti–SARS-CoV-2 antibodies at day 60.
Interpretation
There was no mortality benefit with tocilizumab through day 60. Tocilizumab did not impair viral clearance or host immune response, and no new safety signals were observed. Future investigations may explore potential biomarkers to optimize patient selection for tocilizumab treatment and combination therapy with other treatments.
Funding
F. Hoffmann-La Roche Ltd and the US Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under OT number HHSO100201800036C.
Keywords: Coronavirus disease 2019, Interleukin-6, Randomised controlled trial, Tocilizumab, Severe acute respiratory syndrome coronavirus-2, Viral load
Research in context.
Evidence before this study
We searched PubMed from 1 January 2020, through 23 September 2021, using the term “tocilizumab AND COVID-19” limited to randomised controlled trials. Among all trials, a survival benefit was demonstrated in two platform trials, REMAP-CAP and RECOVERY. A published meta-analysis of data from 19 clinical trials of tocilizumab in patients hospitalised with COVID-19, including REMAP-CAP and RECOVERY, reported an absolute mortality risk of 22% for tocilizumab (n = 4299) compared with an assumed mortality risk of 25% for usual care or placebo (n = 3749), which corresponded to a summary odds ratio of 0.83 (95% CI, 0.74 to 0.92). No randomised controlled trials have reported the effect of tocilizumab on viral load or on the development of anti–Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) antibodies.
Added value of this study
This study provides clinically important data about the efficacy and safety of tocilizumab in patients hospitalised with COVID-19 pneumonia past the 28 days of observation reported in previous trials and evaluates the efficacy of tocilizumab across subgroups of patients defined by baseline inflammatory markers, SARS-CoV-2 viral load, standard-of-care medications, and patient characteristics. Additional analysis of the effect of tocilizumab on viral clearance and host immune response that was not investigated in the REMAP-CAP and RECOVERY trials demonstrates that treatment with tocilizumab did not delay SARS-CoV-2 viral clearance or affect the development of neutralising antibodies.
Implications of all the available evidence
The evidence to date suggests that treatment with tocilizumab provides some benefits beyond standard care. Future investigations are needed to confirm appropriate patient selection and to evaluate outcomes when tocilizumab is used in combination with other treatments. Tocilizumab treatment appears to be safe and does not delay viral clearance or affect the development of anti–SARS-CoV-2 antibodies.
Alt-text: Unlabelled box
Introduction
Two large, randomised, controlled platform trials, RECOVERY and REMAP-CAP, demonstrated survival benefit with tocilizumab, an interleukin-6 (IL-6) receptor blocker, compared with standard care alone at day 28.1,2 This was confirmed in a meta-analysis that included data from 19 clinical trials of tocilizumab in patients hospitalised with coronavirus disease-19 (COVID-19).3 Randomised controlled trials of tocilizumab in COVID-19 have provided heterogeneous results, possibly because of differences in patient selection, timing of treatment, sample sizes, and rapidly evolving standard care.4, 5, 6
Potential impacts of IL-6 blockade on Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) viral clearance and neutralising anti–SARS-CoV-2 antibody development are important considerations in assessing the overall benefits and risks of tocilizumab in the treatment of COVID-19.7 SARS-CoV-2 viral load is correlated with elevated IL-6 levels and poor disease outcomes.8, 9, 10, 11 However, data are needed from randomised controlled trials to determine whether tocilizumab impacts viral clearance or affects the humoural immune response.
COVACTA was a randomised, double-blind, placebo-controlled trial of tocilizumab in hospitalised patients with COVID-19. The primary outcome of COVACTA (clinical status assessed on a 7-category ordinal scale at day 28) was not significantly different between tocilizumab and placebo.12 Although there was no difference in mortality between tocilizumab and placebo at day 28 in COVACTA, potential benefits in time to discharge/ready for discharge and duration of intensive care unit (ICU) stay were identified.12 Here, we report on the efficacy and safety of tocilizumab through day 60 in COVACTA and describe the effects of tocilizumab on SARS-CoV-2 load and neutralising antibody titres. To our knowledge, this is the first report of the longer-term efficacy and safety of IL-6 inhibition in COVID-19 and the first report of virology and serology data from a large, randomised, placebo-controlled trial.
Methods
Study design and patients
COVACTA (ClinicalTrials.gov, NCT04320615) was a global, randomised, double-blind, placebo-controlled, phase 3 trial of tocilizumab compared with placebo in patients hospitalised with severe COVID-19 pneumonia. The study design and full enrolment criteria have been published.12 Briefly, adults who had SARS-CoV-2 infection based on local polymerase chain reaction (PCR) testing and were hospitalised because of COVID-19 pneumonia—with blood oxygen saturation of ≤93% or partial pressure of oxygen/fraction of inspired oxygen of <300 mm Hg—were eligible.
Informed consent was obtained for all enrolled patients. The study was conducted in accordance with the International Council for Harmonisation E6 guideline for good clinical practice and the Declaration of Helsinki or local regulations, whichever afforded greater patient protection. The protocol (supplement 1) was reviewed and approved by the institutional review board or the ethics committee at each site.
Randomisation and masking
Patients were randomly assigned in a 2:1 ratio to receive intravenous tocilizumab 8 mg/kg (maximum 800 mg) or placebo plus local standard care (could have included antiviral therapy or corticosteroids in addition to supportive care) using an interactive voice or web-based response system and permuted-block randomisation. A second dose of tocilizumab or placebo could be given within 8 to 24 h after the first dose if clinical signs and symptoms did not improve. Randomisation was stratified by region (North America, Europe) and mechanical ventilation status (yes, no). The study sponsor, site personnel, and patients were masked to treatment assignment and pharmacokinetic and pharmacodynamic measures during the study.
Clinical outcome measures
Day 60 efficacy and safety outcomes and time to clinical improvement to day 28 (secondary outcome not previously published) are reported here.
Clinical status was assessed on a 7-category ordinal scale (1, discharged/ready for discharge; 2, non–ICU hospital ward/ready for hospital ward, not requiring supplemental oxygen; 3, non–ICU hospital ward/ready for hospital ward, requiring supplemental oxygen; 4, ICU or non–ICU hospital ward, requiring noninvasive ventilation or high-flow oxygen; 5, ICU, requiring intubation and mechanical ventilation; 6, ICU, requiring extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; 7, death). ‘Ready for discharge’ was defined as normal body temperature and respiratory rate and stable oxygen saturation on ambient air or ≤2 L supplemental oxygen. Time to clinical improvement up to day 28 was defined as time from initial study treatment to a National Early Warning Score 2 (NEWS2) of ≤2 maintained for 24 h. Incidences of safety events were summarised through day 60.
Laboratory outcome measures
IL-6 was measured by qualified immunoassay (Quantikine® ELISA, R&D Systems at Quest Pharmaceutical Services), and C-reactive protein (CRP) was measured using a validated in vitro diagnostic method (Roche Cobas®, Roche Diagnostics at PPD®) at central laboratories. Ferritin was measured using standard laboratory methods available at local hospital laboratories. Virology and neutralising antibody assays were performed by Viroclinics Biosciences‒DDL Diagnostic Laboratory (Rotterdam, Netherlands). SARS-CoV-2 viral load (RNA copies/μL) was measured by N1-gene quantitative reverse transcriptase PCR (RT-qPCR) in nasopharyngeal/oropharyngeal swabs and serum samples as described,13 with adjusted probe dyes and PCR program and a limit of quantification (LOQ) of 0.12 copies/μL. Change from baseline in viral load was assessed over time as a safety objective. Time to negative PCR result was assessed. Anti–SARS-CoV-2 neutralising antibody titres were measured by plaque reduction neutralisation test (PRNT80)14 in serum samples at baseline, day 28, and day 60. Given that tocilizumab treatment for autoimmune diseases is associated with neutropenia, thrombocytopenia, and elevated levels of liver enzymes,15 relevant laboratory results were monitored locally through day 60.
Statistical analysis
Efficacy was assessed in the modified intention-to-treat (mITT) population, defined as all randomly assigned patients who received any amount of study medication grouped according to treatment assigned at randomisation. Time to clinical improvement to day 28 was assessed using a log-rank test and a Cox proportional hazards model stratified by region and mechanical ventilation status at randomisation, and Kaplan-Meier plots were produced. Clinical improvement criteria were met if a patient had at least two assessments within a 24 h (±2·5 h) period with no NEWS2 score >2 or if they had a score ≤2 and were then discharged within 26·5 h with no score >2 before discharge. The NEWS2 score was not calculated if one of the components was missing at a particular time point. Hazard ratios for the post hoc subgroup analyses of time to death were estimated using the Cox proportional hazards model (unstratified). Subgroups for the time to death analyses were based on demographics, disease characteristics and viral load, standard of care medications, comorbidities, and inflammatory markers at baseline. Difference in the duration of supplemental oxygen was assessed using the van Elteren test stratified by region and mechanical ventilation status at randomisation, and cumulative distribution plots were produced. Patients who died by day 28 were assigned a duration of supplemental oxygen of 28 days. Mortality at day 60 was summarised (prespecified) and analysed (post hoc) using the Cochran-Mantel-Haenszel test stratified by region and mechanical ventilation status at randomisation. A conditional logistic regression analysis modeling the probability of death at day 60 was conducted as an additional sensitivity analysis. The proportions of patients who required supplemental oxygen at or after discharge were summarised. Time to death through day 60 was analysed post hoc. No multiplicity adjustments were made for p values or 95% CIs.
Post hoc sensitivity analyses were conducted for time to clinical improvement to day 28, mortality at day 60, and duration of supplemental oxygen to day 28 that were adjusted for age and corticosteroid use at baseline in addition to the stratification factors of region and mechanical ventilation status at randomization included in the original stratified analysis.
Safety (including virology, serology, and laboratory data) was assessed in the safety-evaluable population, which was defined as all randomly assigned patients who received any study medication grouped according to the treatment first received rather than the treatment assigned. Time to first negative PCR result from nasopharyngeal/oropharyngeal swabs (prespecified) and serum samples (post hoc) was assessed using a Cox proportional hazards model stratified by region and mechanical ventilation at randomisation in the subgroup of patients with positive test results at baseline, and Kaplan-Meier plots were produced. The area under the curve (AUC) of SARS-CoV-2 viral load was calculated post hoc using the trapezoidal method adjusted by the date and time of the last available assessment for each patient. For AUC calculations and summaries of viral load over time, values from samples with viral loads below the LOQ were imputed to the LOQ minus 0·001 (0·119 copies/μL), and negative samples were imputed to LOQ divided by 2 (0·06 copies/μL). Anti–SARS-CoV-2 neutralising antibody titers were summarised post hoc.
Role of the funding source
The study was funded by F. Hoffmann-La Roche Ltd (the sponsor) and, in part, by federal funds received from the US Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under OT number HHSO100201800036C. The sponsor was involved in the design and conduct of the study, analysis and interpretation of the data, review and critical revision of the manuscript, and decision to submit the paper for publication. All authors had full access to all the data reports and final responsibility to submit the manuscript for publication.
Results
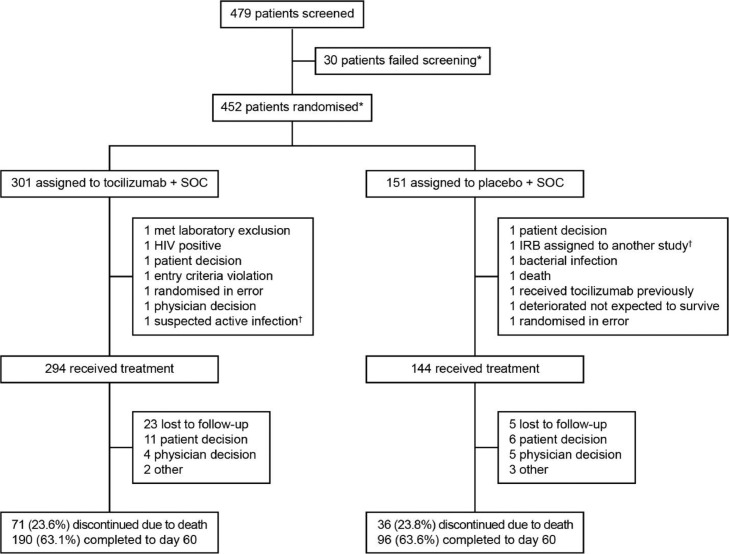
Overall, 452 patients were enrolled at 62 centres across nine countries in Europe and North America between 3 April 2020 and 28 May 2020. Of these, 438 received study treatment and were included in the mITT population. Patient demographics and disease characteristics at baseline were generally well balanced between the treatment groups (Supplementary Table S1).12 Among all randomly assigned patients, 190 (63·1%) in the tocilizumab arm and 96 (63·6%) in the placebo arm completed the study to day 60; death was the most common reason for discontinuation (Figure 1). The total number of patients discharged from hospital to day 60 in the mITT population was 197 (67·0%) in the tocilizumab arm and 92 (63·9%) in the placebo arm (Table 1).
Figure 1.
COVACTA trial profile. *Three patients were rescreened and subsequently randomly assigned. †Patient was randomly assigned again and later received study treatment. Abbreviations: IRB, Institutional Review Board; SOC, standard of care.
Table 1.
Summary of patients discharged over time (modified intention-to-treat population).
| Patients discharged, n (%) | Tocilizumab N = 294 |
Placebo N = 144 |
||
|---|---|---|---|---|
| Weekly | Cumulative | Weekly | Cumulative | |
| Day 7 | 55 (18·7) | 55 (18·7) | 19 (13·2) | 19 (13·2) |
| Day 14 | 58 (19·7) | 113 (38·4) | 24 (16·7) | 43 (29·9) |
| Day 21 | 28 (9·5) | 141 (48·0) | 16 (11·1) | 59 (41·0) |
| Day 28 | 16 (5·4) | 157 (53·4) | 7 (4·9) | 66 (45·8) |
| Day 35 | 9 (3·1) | 166 (56·5) | 7 (4·9) | 73 (50·7) |
| Day 45 | 15 (5·1) | 181 (61·6) | 13 (9·0) | 86 (59·7) |
| Day 60 | 16 (5·4) | 197 (67·0) | 6 (4·2) | 92 (63·9) |
Only first discharge is shown. Patients who were readmitted to hospital within 12 h were not counted as discharged.
Day 60 outcomes
Through day 60, 36 of 197 patients (18·3%) in the tocilizumab arm and 24 of 92 patients (26·1%) in the placebo arm required supplemental oxygen at or after the time of hospital discharge.
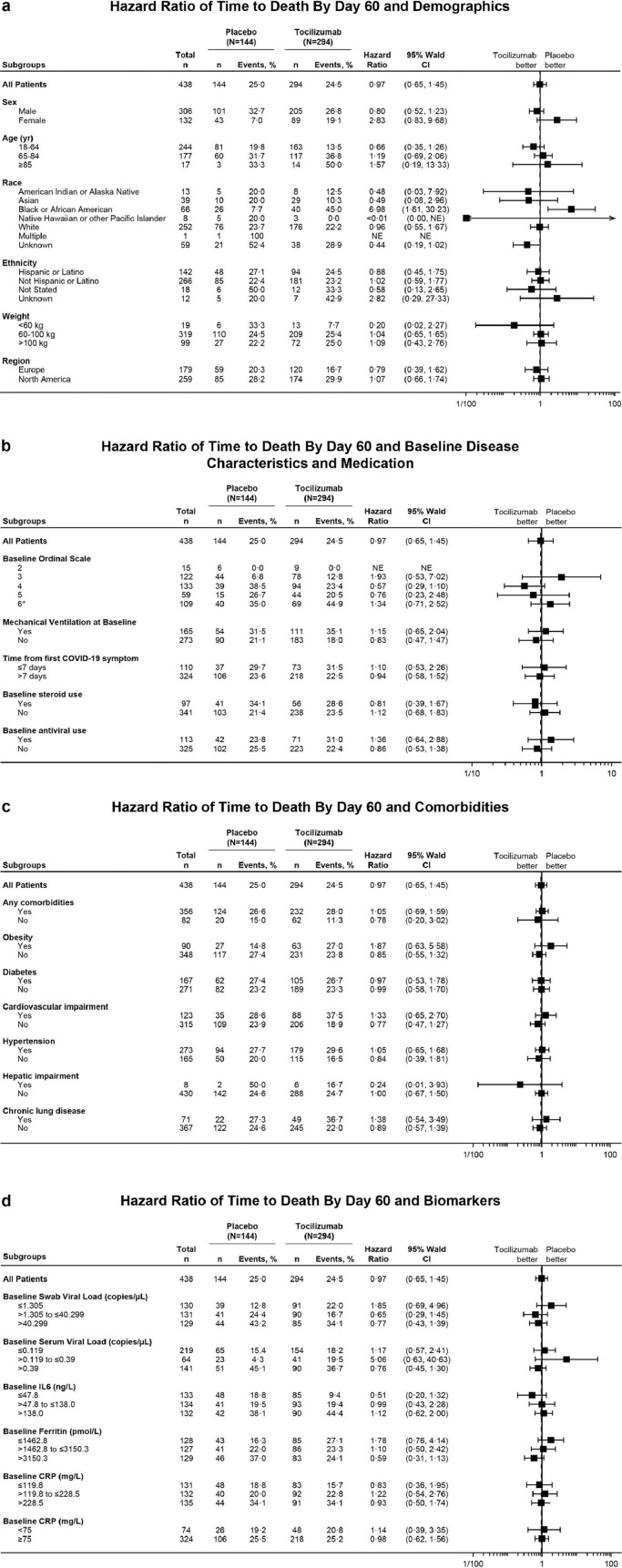
In the mITT population, 72 of 294 patients (24·5%) in the tocilizumab arm and 36 of 144 patients (25·0%) in the placebo arm died by day 60 (weighted difference –0·5% [95% CI –9·1 to 8·0]; Cochran-Mantel-Haenszel p = 0·90 in post hoc analysis). Per a post hoc analysis of time to death by day 60 among subgroups based on baseline demographics, clinical status, viral load, standard-of-care medications, comorbidities, or inflammatory markers (IL-6, ferritin, CRP), all 95% CIs contained the value of 1 except for Black or African American patients (Figure 2a–d). However, data interpretation in all subgroups is limited because of small patient numbers. At baseline, 71 patients (24·1%) in the tocilizumab arm and 42 patients (29·2%) in the placebo arm were receiving antiviral treatments (19 [6·5%] and 6 [4·2%], respectively, were receiving remdesivir; Supplementary Table S2) and 56 (19·0%) and 41 (28·5%), respectively, were receiving systemic corticosteroids. In patients receiving baseline antiviral therapy, the hazard ratio (HR) was 1·36 (95% CI 0·64 to 2·88), and in patients receiving baseline corticosteroids, the HR was 0·81 (95% CI 0·39 to 1·67) (Figure 2b).
Figure 2.
Summary forest plots showing the hazard ratio of time to death by day 60 associated with (a) demographics, (b) baseline disease characteristics and concomitant medications, (c) comorbidities, and (d) biomarkers (mITT population). Hazard ratios were estimated using Cox proportional hazards model (unstratified). A hazard ratio of <1 favoured tocilizumab over placebo. Patients who did not die were censored at study completion or early withdrawal. (b) Baseline ordinal scale refers to clinical status assessed on a 7-category ordinal scale (1, discharged/ready for discharge; 2, non–ICU hospital ward/ready for hospital ward, not requiring supplemental oxygen; 3, non–ICU hospital ward/ready for hospital ward, requiring supplemental oxygen; 4, ICU or non–ICU hospital ward, requiring noninvasive ventilation or high-flow oxygen; 5, ICU, requiring intubation and mechanical ventilation; 6, ICU, requiring extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; 7, death). *Ordinal scale 6 included one patient who was initially in ordinal scale 6 on day 1 but died (ordinal scale 7) on day 1. Baseline steroid or antiviral use was defined as between day –7 and day 1. Steroid treatments included corticosteroids except those reported as topical, inhalant or dermatological. Antiviral treatments included lopinavir/ritonavir, remdesivir, lopinavir, ritonavir, chloroquine, hydroxychloroquine, or hydroxychloroquine sulphate. (c) Any comorbidities include patients with ≥1 comorbidity of obesity, diabetes, cardiovascular impairment, hepatic impairment, hypertension, or chronic lung disease. (d) Baseline nasopharyngeal/oropharyngeal swab or serum viral load was defined as a patient's most recent assessment before the first dose of study medication. If no assessment was available with a time before the first dose of study medication, the assessment labelled as ‘day 1 predose’ assessment was treated as baseline. Baseline IL-6, ferritin, and CRP levels were determined by a patient's most recent pretreatment assessment. Patients whose baseline CRP or ferritin values were above the upper limit of the assay were assigned to the highest category. Patients whose baseline IL-6 or viral load values were below the limit of quantification or were negative (viral load only) were assigned to the lowest category. Abbreviations: CRP, C-reactive protein; ICU, intensive care unit; IL-6, interleukin-6; mITT, modified intention-to-treat; NE, not evaluable.
By day 60, 240 of 295 patients (81·4%) in the tocilizumab arm and 118 of 143 patients (82·5%) in the placebo arm experienced ≥1 adverse event and 116 patients (39·3%) in the tocilizumab arm and 64 patients (44·8%) in the placebo arm experienced ≥1 serious adverse event (Table 2). No events led to withdrawal from the study other than those that resulted in death. The most common serious adverse events were infections; excluding preferred terms COVID-19 and COVID-19 pneumonia, the most common serious infections were septic shock (tocilizumab, 7 [2·4%]; placebo, 7 [4·9%]), pneumonia (tocilizumab, 7 [2·4%]; placebo, 4 [2·8%]), bacterial pneumonia (tocilizumab, 6 [2·0%]; placebo, 2 [1·4%]), and sepsis (tocilizumab, 3 [1·0%]; placebo, 4 [2·8%]). Eight serious infections (excluding COVID-19 and COVID-19 pneumonia) occurred after day 28, four (1·4%) in the tocilizumab arm and four (2·8%) in the placebo arm. In the safety population, 72 patients (24·4%) in the tocilizumab arm and 36 patients (25·2%) in the placebo arm died by day 60.
Table 2.
Adverse events through day 60 (safety population).
| Tocilizumab N = 295 | Placebo N = 143 | |
|---|---|---|
| Adverse events, n | 949 | 433 |
| Patients with ≥1 adverse event, n (%) | 240 (81·4) | 118 (82·5) |
| Percentage difference (95% CI) | −1·2 (−8·4, 7·0) | |
| Serious adverse events, n | 192 | 122 |
| Patients with ≥1 serious adverse event, n (%)* | 116 (39·3) | 64 (44·8) |
| Percentage difference (95% CI) | −5·4 (−15·2, 4·3) | |
| Deaths, n (%)† | 72 (24·4) | 36 (25·2) |
| Percentage difference (95% CI) | −0·8 (−9·7, 7·5) | |
| Patients with ≥1 adverse event of special interest, n (%), percentage difference (95% CI) | ||
| Infections | 127 (43·1) | 63 (44·1) |
| −1·0 (−10·9, 8·7) | ||
| Serious infections‡ | 71 (24·1) | 42 (29·4) |
| −5·3 (−14·4, 3·3) | ||
| Opportunistic infections§ | 1 (0·3) | 3 (2·1) |
| −1·8 (−5·7, 0·3) | ||
| Bleeding events | 47 (15·9) | 18 (12·6) |
| 3·3 (−4·1, 9·8) | ||
| Serious bleeding events | 13 (4·4) | 5 (3·5) |
| 0·9 (−3·9, 4·5) | ||
| Hypersensitivity¶ | 19 (6·4) | 4 (2·8) |
| 3·6 (−1·1, 7·5) | ||
| Anaphylactic reaction according to Sampson's criteria | 0 | 1 (0·7) |
| – | ||
| Hepatic events | 7 (2·4) | 3 (2·1) |
| 0·3 (−3·8, 3·1) | ||
| Malignancies | 1 (0·3) | 0 |
| – | ||
| Medically confirmed malignancies | 1 (0·3) | 0 |
| – | ||
| Stroke | 3 (1·0) | 4 (2·8) |
| −1·8 (−6·0, 0·8) | ||
| Myocardial infarction | 4 (1·4) | 2 (1·4) |
| −0·04 (−3·7, 2·3) | ||
| Gastrointestinal perforation | 1 (0·3) | 2 (1·4) |
| −1·1 (−4·6, 0·8) | ||
| Laboratory abnormalities | ||
| Patients with non-missing baseline ALT assessment and ≥1 post baseline ALT assessment, n ALT level ≥ grade 3, n (%)⁎⁎ Grade 3 Grade 4 |
280 17 (6·1) 13 (4·6) 4 (1·4) |
140 6 (4·3) 5 (3·6) 1 (0·7) |
| Neutrophil count > LLN at baseline, n Neutrophil count ≥ grade 3, n (%)†† Grade 3 Grade 4 |
245 12 (4·9) 9 (3·7) 3 (1·2) |
115 1 (0·9) 1 (0·9) 0 |
| Platelet count >LLN at baseline, n Platelet count ≥ grade 3, n (%)†† Grade 3 Grade 4 |
258 10 (3·9) 7 (2·7) 3 (1·2) |
122 1 (0·8) 1 (0·8) 0 |
| Concurrent elevation of ALT or AST level >3 × ULN and total bilirubin level >2 × ULN, n (%) | 6 (2·0) | 7 (4·9) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID-19, coronavirus disease-19; LLN, lower limit of normal; MedDRA, Medical Dictionary for Regulatory Activities; ULN, upper limit of normal.
Excluding infections, other serious adverse events by MedDRA system organ class that were reported in ≥5% of patients (in either treatment arm) included respiratory, thoracic, and mediastinal disorders (tocilizumab, 23 patients [7·8%]; placebo, 21 patients, [14·7%]) and cardiac disorders (tocilizumab, 16 patients [5·4%]; placebo, 9 patients [6·3%]).
The most common reason for death was COVID-19 pneumonia (36 of 72 deaths in the tocilizumab arm and 20 of 36 deaths in the placebo arm).
Excluding COVID-19 and COVID-19 pneumonia, eight serious infections occurred after day 28, four (urinary tract infection, bacterial sepsis, bacteremia, empyema) in the tocilizumab arm and four (septic shock, staphylococcal pneumonia, osteomyelitis, Pneumocystis jirovecii pneumonia) in the placebo arm.
Candida sepsis in the tocilizumab arm and one event each of Candida sepsis, Pneumocystis jirovecii pneumonia, and respiratory moniliasis in the placebo arm.
Hypersensitivity reactions include all events that occurred during or within 24 h after the infusion of tocilizumab or placebo and that were assessed by the investigator as not unrelated to the infused treatment regardless of whether they were clinically consistent with hypersensitivity.
Percentages based on the number of patients with non-missing baseline assessment and ≥1 post baseline assessment.
Percentages based on the number of patients with levels >LLN for neutrophil and platelet counts at baseline.
The 95% CIs for the percentage differences were estimated using the Newcombe method.
No medically confirmed gastrointestinal perforation or demyelinating adverse events were reported.
Additional day 28 efficacy outcomes
Criteria for clinical improvement (NEWS2 ≤2 for 24 h) were met in 103 patients (35·0%) in the tocilizumab arm and 41 patients (28·5%) in the placebo arm by day 28. Median time to clinical improvement was not evaluable in either treatment arm (log-rank p = 0·044; Cox proportional hazards ratio 1·45 [95% CI 1·01 to 2·08]) (Supplementary Figure S1a). The median (95% CI) duration of supplemental oxygen through day 28 was 26·5 days (19·0 to 28·0) in the tocilizumab arm and 28·0 days (26·0 to 28·0) in the placebo arm, with a difference of –1·5 days (–9·0 to 0·5) (van Elteren p = 0·048). The cumulative distribution of the duration of supplemental oxygen to day 28 is shown in Supplementary Figure S1b.
Virology and serology outcomes
SARS-CoV-2 viral load in nasopharyngeal swab samples
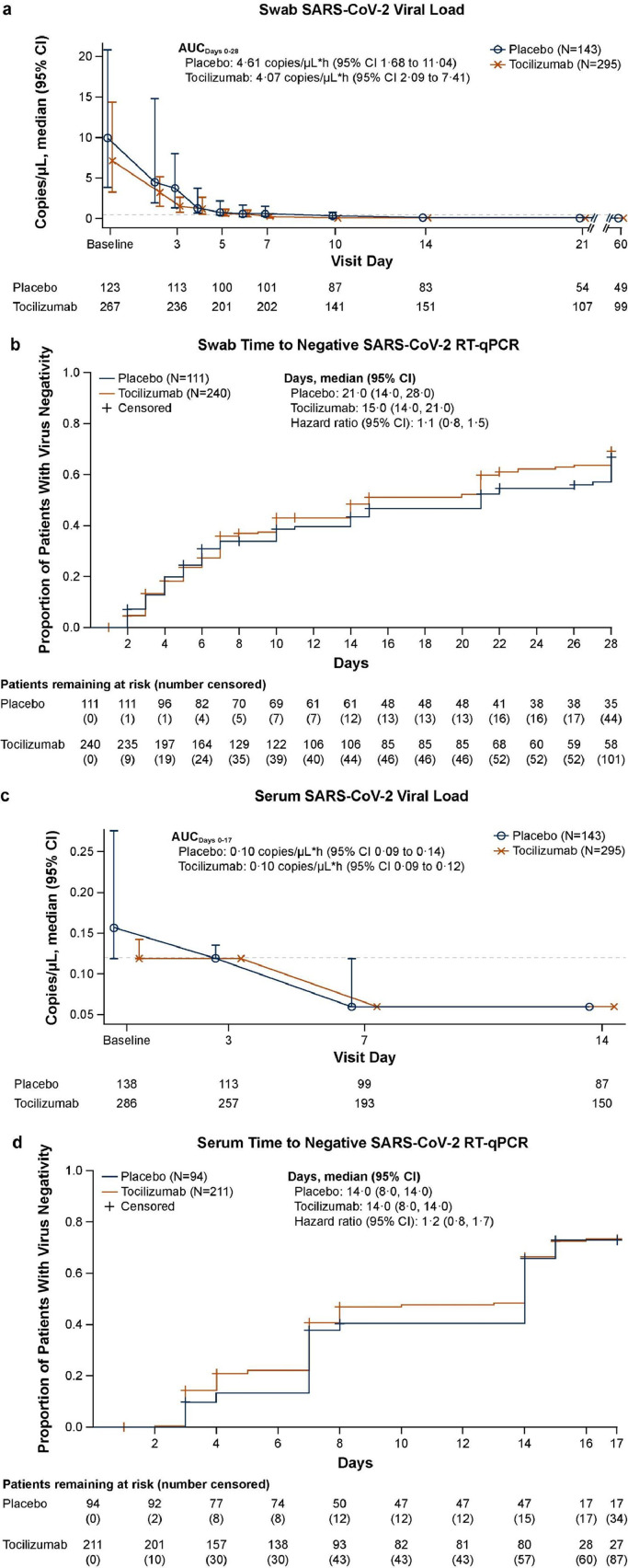
Although all randomly assigned patients except one had a positive test result for SARS-CoV-2 based on local RT-PCR at screening, 351 of 391 patients (89·8%) with baseline assessments had positive RT-qPCR central laboratory results for SARS-CoV-2 based on nasopharyngeal/oropharyngeal samples (240/268 patients [89·6%] in the tocilizumab arm and 111/123 patients [90·2%] in the placebo arm). Median viral loads were 7·17 copies/μL (range 0·1 to 94,385·3) in the tocilizumab arm and 9·89 copies/μL (range 0·1 to 58,450·4) in the placebo arm, decreasing to 0·24 copies/μL (range 0·1 to 31,584·9) and 0·62 copies/μL (range 0·1 to 109,940·6), respectively, by day 7 (Figure 3a). Median AUC by day 28 was 4·07 copies/μL·hour (95% CI 2·09 to 7·41) in the tocilizumab arm and 4·61 copies/μL·hour (95% CI 1·68 to 11·04) in the placebo arm.
Figure 3.
Viral load from (a) swab and (c) serum samples over time and time to first negative RT-qPCR result in (b) swab and (d) serum samples in patients with positive test results at baseline (safety population). (a, b) Nasopharyngeal or oropharyngeal swab samples. (a, c) Data shown are median (95% CI). Horizontal dashed line represents the LOQ of 0.12 copies/μL. Any values reported as below the LOQ were set to the LOQ value minus 0.001 (0.119 copies/μL), and any values reported as negative were set to half the LOQ value (0.06 copies/μL). Baseline is the last pretreatment assessment. If no assessment was available with a time before the first dose of study medication, the assessment labelled as ‘day 1 predose’ assessment was treated as baseline. The AUC was calculated post hoc using the trapezoidal method adjusted by the date and time of the last available assessment of each patient. (b, d) Data are shown as 1 minus the Kaplan-Meier estimator. Time to negative RT-qPCR result was defined as days from the first dose of study drug to time of negative RT-qPCR result in swab or serum samples. Only patients with ≥1 virology assessment were included. Patients who discontinued the study or were lost to follow-up before a virus negativity result were censored at their last virology assessment. Patients who died were censored at day 28 (swab samples) or day 17 (serum samples). Cox proportional hazards model stratified by region and mechanical ventilation at randomisation. Abbreviations: AUC, area under the curve; CI, confidence interval; LOQ, limit of quantification; RT, qPCR-reverse transcriptase–quantitative polymerase chain reaction; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Among patients with a positive RT-qPCR result at baseline, the median time to negative RT-qPCR was 15·0 days (95% CI 14·0 to 21·0) in the tocilizumab arm and 21·0 days (95% CI 14·0 to 28·0) in the placebo arm. The HR for time to negative RT-qPCR with tocilizumab vs. placebo was 1·13 (95% CI 0·83 to 1·53) (Figure 3b). By day 28, 139/240 patients (57·9%) in the tocilizumab arm and 67/111 patients (60·4%) in the placebo arm had achieved RT-qPCR negativity. However, the overall median viral load at baseline was numerically lower in the tocilizumab arm than in the placebo arm. In a post hoc analysis adjusted for baseline viral load and time from symptom onset among patients in the safety population who had a positive baseline RT-qPCR result, the median time to negative RT-PCR result was the same as in the original analysis and the Cox proportional hazard ratio was 1·05 (95% CI, 0·77 to 1·43).
SARS-CoV-2 viral load in serum samples
The median RT-qPCR SARS-CoV-2 viral load was lower in serum samples than in upper respiratory samples at corresponding time points. At baseline, the proportion of patients with a positive serum SARS-CoV-2 RT-qPCR result among those who had a baseline assessment was 73·3% (211/288) in the tocilizumab arm and 67·1% (94/140) in the placebo arm. By day 14, the proportion of patients with a positive serum RT-qPCR result had declined to 17·2% (26/151) in the tocilizumab arm and 9·2% (8/87) in the placebo arm. The median viral load in serum samples was 0·12 copies/μL in the tocilizumab arm and 0·16 copies/μL in the placebo arm at baseline, and by day 7 and day 14 more than half the patients in each arm had negative test results (Figure 3c).
Among patients with a positive serum RT-qPCR result at baseline, the median time to RT-qPCR negativity was 14·0 days in both treatment arms (95% CI 8·0 to 14·0). The HR for time to RT-qPCR negativity in the tocilizumab arm compared with the placebo arm was 1·17 (95% CI 0·82 to 1·68) (Figure 3d). By day 17, 124 of 211 patients (58·8%) in the tocilizumab arm and 60 of 94 patients (63·8%) in the placebo arm had achieved RT-qPCR negativity. In a post hoc analysis adjusted for baseline viral load and time from symptom onset among patients in the safety population who had a positive baseline RT-qPCR result, the median time to negative RT-PCR result was the same as in the original analysis and the Cox proportional hazard ratio was 0·97 (95% CI, 0·66 to 1·42). Among patients with a positive serum RT-qPCR result at baseline who did not receive systemic corticosteroids or remdesivir at baseline or at any time during the study, the median time to negative RT-qPCR result was 14·0 days (95% CI 8·0 to 14·0; n = 121) in the tocilizumab arm and 14·0 days (95% CI, 7·0 to 14·0; n = 36) in the placebo arm (Cox proportional hazards ratio 0·81 [0·46 to 1·44]). Among these patients, 67 (55·4%) in the tocilizumab arm and 22 (61·1%) in the placebo arm achieved RT-qPCR negativity by day 17.
Neutralising anti–SARS-CoV-2 antibodies in serum samples
Regarding serology, 209 of 237 patients (88·2%) in the tocilizumab arm and 95 of 108 patients (88·0%) in the placebo arm had positive test results for neutralising anti–SARS-CoV-2 antibodies at baseline, with median PRNT80 titres of 85·0 (8·0 to 3973·0) and 81·0 (8·0 to 1240·0), respectively. At day 28, 100% of tested patients in the tocilizumab arm and 98·3% of tested patients in the placebo arm had positive test results for neutralising anti–SARS-CoV-2 antibodies. At day 60, all tested patients had positive test results for neutralising antibodies, and median PRNT80 titres had increased to 108·0 (21·0 to 847·0) in the tocilizumab arm and to 189·5 (20·0 to 776·0) in the placebo arm.
Discussion
COVACTA did not meet its primary endpoint of clinical status assessed on an ordinal scale and did not show improvement in mortality; however, initial results suggested that tocilizumab may reduce the time to discharge/ready for discharge and the duration of ICU stay compared with standard care alone.12 The data presented here show potential benefits of tocilizumab in time to clinical improvement and supplemental oxygen use to day 28. Post hoc analysis of time to death to day 60 showed no difference between tocilizumab and placebo overall or by subgroups of baseline demographics, clinical status, standard-of-care medications, comorbidities, viral load, or inflammatory markers. The safety of tocilizumab through day 60 was consistent with that reported through day 28, with no new safety signals. Tocilizumab did not negatively impact viral clearance in the upper respiratory tract or serum or affect the humoural immune response to SARS-CoV-2.
Because IL-6 is a driver of inflammation, it has been postulated that IL-6 receptor inhibition may provide benefit in severe, progressive disease when local and systemic inflammation are the major drivers of disease and before irreversible multiorgan failure occurs.7 Investigators have proposed using inflammatory markers such as CRP, IL-6, and ferritin to select patients most likely to benefit from treatment with IL-6 receptor inhibition, and other studies of tocilizumab in patients with COVID-19 include elevated levels of inflammatory biomarkers as eligibility criteria. In an exploratory analysis of the COVACTA trial, several biomarkers of hyperinflammation, macrophage activation, and dysregulated immune cells were prognostic for clinical outcomes; however, only ferritin was predictive for the effects of tocilizumab.16 The data presented here show no statistically significant differences in time to death through day 60 in subgroups based on tertiles of serum CRP, IL-6, or ferritin levels or based on CRP level lower or higher than 75 mg/L at baseline, which was a criterion for enrolment in the RECOVERY tocilizumab cohort.2 It has been suggested that local inflammation manifesting as rapidly worsening respiratory dysfunction, rather than systemic inflammatory markers such as CRP and IL-6 in peripheral blood, might be a more useful indicator of which patients are likely to benefit from IL-6 inhibition. Furthermore, prespecified analysis revealed the consistent efficacy of tocilizumab across subgroups of patients with different serum CRP levels at baseline in the REMAP-CAP trial.1
Efficacy results from COVACTA should be interpreted in the context of other clinical trials of tocilizumab in COVID-19. Some trials that included moderately to severely ill patients did not demonstrate consistent efficacy of tocilizumab above standard care.17, 18, 19 The REMAP-CAP trial recruited critically ill patients within 24 h of initiation of organ support and demonstrated that tocilizumab reduced the need for ongoing organ support and conferred a survival benefit compared with standard care.1 RECOVERY, a large, randomised, open-label, platform trial in more than 4000 patients, showed that treatment with tocilizumab significantly reduced the risk of death by day 28 compared with standard care in hospitalised patients with COVID-19, regardless of the type of respiratory support. Meta-analyses have demonstrated that tocilizumab reduced all-cause mortality by day 28 compared with standard care alone.2,3,20 The COVACTA population spanned a broad spectrum, from patients requiring low-flow oxygen to patients requiring mechanical ventilation and experiencing multiorgan failure at enrolment. The heterogeneous population and evolving background treatment (local standard care), combined with insufficient sample size to detect a mortality difference of the magnitude seen in RECOVERY, may explain the inconsistent day 28 mortality findings in COVACTA.5 Results of RECOVERY also suggest that the largest benefit of tocilizumab may be in patients receiving corticosteroids.2 In COVACTA, 22% of patients were receiving systemic corticosteroids at baseline; in REMAP-CAP and in RECOVERY, respectively, 93% received dexamethasone and 82% received corticosteroids.1,2 The results of post hoc sensitivity analyses adjusted for age and corticosteroid use at baseline in addition to the stratification factors in the original stratified analysis were generally consistent with the original analyses (Supplementary Tables S3–S6).
SARS-CoV-2 viral load in the upper respiratory tract peaks during the first week of infection,21 and neutralising SARS-CoV-2 antibodies can accelerate the subsequent decline in viral load.22 Stronger immune response and higher levels of neutralising SARS-CoV-2 antibodies are detected in patients with severe disease than in those with mild disease,23 and viral load is higher in severe COVID-19.10 In addition, SARS-CoV-2 RNA is detected in the sera of the most critically ill patients.8 A prospective cohort study suggested that delayed viral clearance was attributable to a higher baseline viral load in 76 patients hospitalised with COVID-19 treated with tocilizumab, but the specific antibody response to SARS-CoV-2 was not impaired.24 Our study is the first, to our knowledge, to show in a double-blind, placebo-controlled, randomised trial that tocilizumab does not negatively impact viral clearance or affect the humoural immune response to SARS-CoV-2. These results may have implications for guiding prevention and control strategies for infection. In the tocilizumab and placebo arms of COVACTA, the time to viral load negativity in upper respiratory tract samples was comparable in patients who were SARS-CoV-2 RT-qPCR‒positive at baseline. Similar results were observed for serum viral load, confirming consistent viral clearance between treatment arms. Furthermore, there was no difference observed between tocilizumab and placebo in the development of postbaseline neutralising anti–SARS-CoV-2 antibodies, although neutralising anti–SARS-CoV-2 antibodies were detected in most patients at baseline, likely reflecting the overall late/severe stage of COVID-19 disease in the study population.
Limitations of this study include the heterogeneous patient population and the small sample size compared with other trials. The small sample size was a particular limitation for subgroup analyses because it did not allow the analyses to be stratified. Data interpretation is also limited in the subgroup analyses because no multiplicity adjustments were made for the 95% CIs. The primary endpoint was chosen in consultation with health authorities because it integrates several outcomes that are potentially important in the context of the pandemic. Subsequent studies, however, have focused on different endpoints.1,2,25 The small sample size in this study meant that it was not sufficiently powered to detect a difference in mortality that was reported in larger platform trials; this limits the interpretation of mortality results and associated subgroup analyses. Furthermore, there were no adjustments for multiplicity in our study. Clinical improvement was assessed using the strict NEWS2 criteria as a conservative approach to support other efficacy endpoints. Some patients were discharged from the hospital without meeting these criteria, which limited the number of patients with clinical improvement: 41 patients (28·5%) in the placebo arm and 103 patients (35·0%) in the tocilizumab arm met the NEWS2 criteria for clinical improvement. All patients in this study except one tested positive for SARS-CoV-2 at baseline according to local RT-PCR tests; 351 of 391 patients (89·8%) who had a baseline nasopharyngeal or oropharyngeal swab sample had a positive result in central testing. This small discordance is unlikely to have affected the results of the study because the proportion of patients who were negative according to central testing was low and was balanced across the treatment arms (12/123 patients [9·8%] in the placebo arm and 28/268 [10·4%] in the tocilizumab arm).
In conclusion, there was no mortality benefit with tocilizumab treatment observed by day 60 in COVACTA. Compared with placebo, tocilizumab treatment did not delay the clearance of SARS-CoV-2 or the development of neutralising antibodies. Overall, the safety profiles were balanced between the tocilizumab and placebo arms through day 60.
Contributors
Substantial contributions to the conception or design of the work; and the acquisition, analysis, interpretation of data for the work: IOR, NB, MW, AM, BDH, SB, DS, SS, MSA, TY, DJDLZ, ACG, KGB, JC, BF, TB, DH, NR, CEL, RNB, FC, ITL, BM, LM, SW, EG, LT, MB
Drafting the work or revising it critically for important intellectual content: IOR, NB, MW, RCG, AM, BDH, SB, DS, SS, ISD, JGD, MSA, NC, TY, LDS, DJDLZ, AU, ACG, KGB, JC, BF, TB, PB, CHVDL, NR, RNB, FC, ITL, BM, LM, SW, EG, LT, MB
Final approval of the version to be published: IOR, NB, MW, RCG, AM, BDH, SB, DS, SS, ISD, JGD, MSA, NC, TY, LDS, DJDLZ, AU, ACG, KGB, JC, BF, TB, DH, PB, CHVDL, NR, LW, CEL, RNB, FC, ITL, BM, LM, SW, EG, LT, MB
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: IOR, NB, RCG, BDH, SB, SS, ISD, JGD, MSA, NC, TY, DJDLZ, ACG, KGB, TB, CHVDL, NR, FC, ITL, BM, LM, SW, EG, LT, MB
Funding
F. Hoffmann-La Roche Ltd and the US Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under OT number HHSO100201800036C.
Declaration of interests
Ivan O. Rosas: Grant from Roche/Genentech during the conduct of the study; grant and personal fees from Genentech outside the submitted work; and personal fees from Boehringer and Bristol Myers Squibb outside the submitted work
Norbert Bräu: Grant support to institution from Roche/Genentech during the conduct of the study; grants from Gilead Sciences outside the submitted work
Ronaldo C. Go: Consulting fees from F. Hoffmann-La Roche outside the submitted work
Atul Malhotra: Grants from the National Institutes of Health; personal fees from LivaNova, Corvus, and Equillium; institutional funding from RedMed outside the submitted work
Bradley D. Hunter: Personal fees from Kite Pharmaceuticals and Novartis outside the submitted work
Sanjay Bhagani: Grants and personal fees from Gilead Sciences, Roche, and ViiV outside the submitted work
Sinisa Savic: Grants and personal fees from Novartis and SOBI outside the submitted work
Ivor S. Douglas: Grant support to institution from Roche/Genentech during the conduct of the study
Andrew Ustianowski: Grant support to institution from Roche/Genentech during the conduct of the study
Jordi Carratalà: Grant from Roche/Genentech during the conduct of the study; grant and personal fees from Gilead Sciences outside the submitted work
Thomas Benfield: Grants from Novo Nordisk Foundation, Simonsen Foundation, GlaxoSmithKline, Pfizer, Gilead, Lundbeck Foundation, Kai Hansen Foundation and Erik and Susanna Olesen's charitable fund; personal fees from GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Gilead, MSD and PentaBase A/S outside the submitted work
Paolo Bonfanti: Personal fees from ViiV, Gilead, Janssen, and Merck outside the submitted work.
Cor H. van der Leest: Personal fees related to the submitted work; personal fees from Bristol Myers Squib, Merck Sharp & Dohme, AbbVie, Boehringer Ingelheim, Roche, and AstraZeneca outside the submitted work
Charles Edouard Luyt: Grant support to institution from Roche/Genentech during the conduct of the study; personal fees from Carmat, Merck, bioMérieux, Thermo Fischer Brahms, Bayer Healthcare, and Faron outside the submitted work
Rebecca N. Bauer: Employee of Genentech and holder of stock/stock options in Roche
Fang Cai: Former employee of Genentech; patent pending to Genentech for biomarkers for predicting response to an IL-6 antagonist (P36367-US)
Ivan T. Lee: Former clinical research fellow of Genentech/Roche; received funding from Genentech/Roche during the conduct of the study
Balpreet Matharu: Employee of Roche Products Ltd
Louis Metcalf: Employee of and owns shares in Roche
Steffen Wildum: Employee of F Hoffmann-La Roche
Emily Graham: Employee of Roche Products Ltd
Larry Tsai: Employee of Genentech/Roche; unpublished patent pending for ‘Method for treating pneumonia, including COVID-19 pneumonia, with an IL-6 antagonist’
Min Bao: Grant from Biomedical Advanced Research and Development Authority (BARDA) for the COVACTA study; employee of Genentech/Roche; unpublished patent pending for ‘Method for treating pneumonia, including COVID-19 pneumonia, with an IL-6 antagonist’
All other authors have nothing to disclose.
Acknowledgments
Acknowledgements
We thank the patients and staff who participated in this study. Medical writing assistance was provided by Sara Duggan, PhD, of ApotheCom and was funded by F Hoffmann-La Roche Ltd.
Data sharing statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/) upon publication. Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101409.
Appendix. Supplementary materials
References
- 1.Gordon A.C., Mouncey P.R., Al-Beidh F., et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recovery Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar-Hari M., Vale C.L., Godolphin P.J., for WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326(6):499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angriman F., Ferreyro B.L., Burry L., et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir Med. 2021;9(6):655–664. doi: 10.1016/S2213-2600(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S., Leaf D.E. Tocilizumab in COVID-19: some clarity amid controversy. Lancet. 2021;397:1599–1601. doi: 10.1016/S0140-6736(21)00712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosas I.O., Diaz G., Gottlieb R.L., et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med. 2021;47:1258–1270. doi: 10.1007/s00134-021-06507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Zhao B., Qu Y., et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y., Chen S., Yang Z., et al. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med. 2020;201:1435–1438. doi: 10.1164/rccm.202003-0572LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Yan L.M., Wan L., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fajnzylber J., Regan J., Coxen K., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosas I.O., Bräu N., Waters M., et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X., Wang L., Sakthivel S.K., et al. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1654–1665. doi: 10.3201/eid2608.201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zielinska E., Liu D., Wu H.Y., Quiroz J., Rappaport R., Yang D.P. Development of an improved microneutralization assay for respiratory syncytial virus by automated plaque counting using imaging analysis. Virol J. 2005;2:84. doi: 10.1186/1743-422X-2-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Actemra Prescribing Information. 2021 1. [Google Scholar]
- 16.Tom J., Bao M., Tsai L., et al. Prognostic and predictive biomarkers in patients with coronavirus disease 2019 treated with tocilizumab in a randomized controlled trial. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvarani C., Dolci G., Massari M., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone J.H., Frigault M.J., Serling-Boyd N.J., et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosn L., Chaimani A., Evrenoglou T., et al. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;3 doi: 10.1002/14651858.CD013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cevik M., Tate M., Lloyd O., et al. SARS-CoV-2, SARS-CoV-1 and MERS-CoV viral load dynamics, duration of viral shedding 2 and infectiousness – a living systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Zhang L., Sang L., et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020;130:5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masiá M., Fernández-González M., Padilla S., et al. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: a prospective cohort study. EBioMedicine. 2020;60 doi: 10.1016/j.ebiom.2020.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.