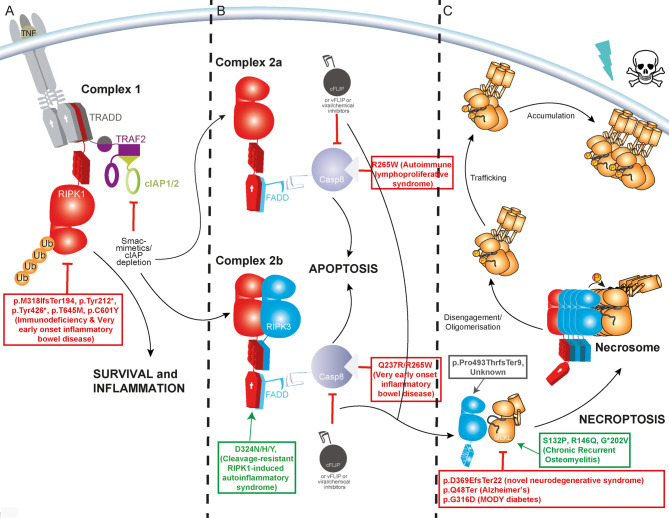
Figure 1. TNF-induced necroptosis occurs when upstream pro-survival and pro-apoptotic pathways are inhibited.
(A) The binding of TNF to TNFR1 stimulates downstream nuclear factor-κB activation and other pro-survival, proinflammatory signals. (B) When cIAP1/2 activity is low, signalling is diverted to the formation of a death-induced signalling complex termed Complex II (a/b). This physically distinct complex is variably composed of TRADD, FADD, RIPK1, RIPK3 and the apoptosis initiator Caspase-8. (C) Necroptosis is activated when cellular conditions curb Caspase-8 activity and favour the assembly of RIPK1 and RIPK3, via their RIP Homotypic Interaction Motif (RHIM) domains, into a high molecular mass complex termed the necrosome. Here, RIPK3 is activated by autophosphorylation and MLKL is recruited and phosphorylated by RIPK3. MLKL dissociates from RIPK3 and oligomerised MLKL is trafficked to biological membrane [84,85]. The precise molecular events that lead to lytic permeabilization of the cell are still a matter of contention [86]. Sites of some selected published human germline mutations shown to be associated with human disease due to gain (red) or loss/reduction in function (green) are shown.