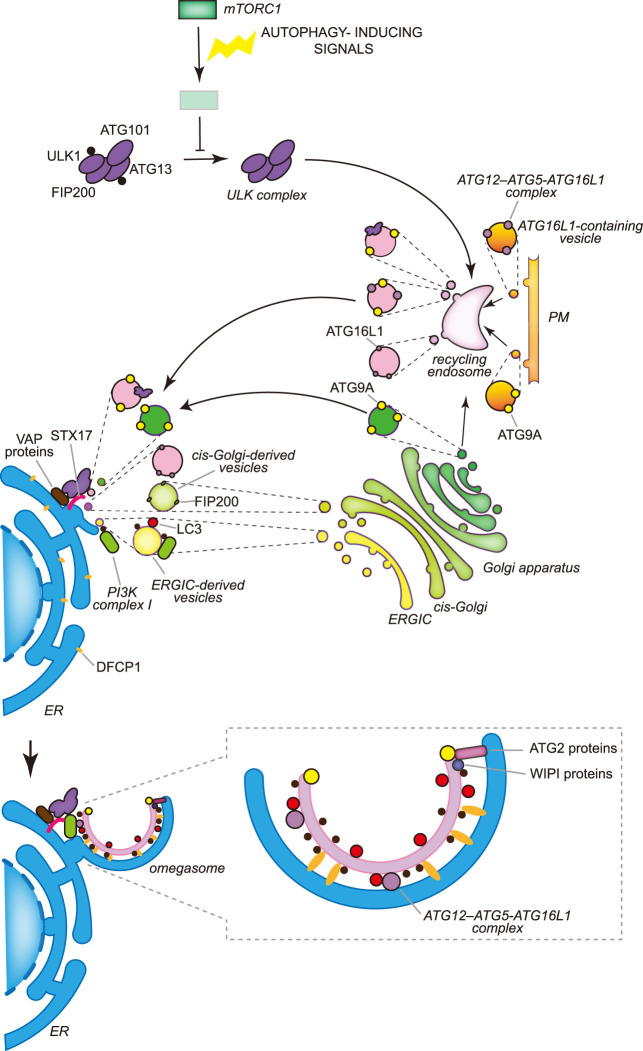
Figure 3. The molecular mechanisms of the early stages of autophagosome biogenesis during bulk autophagy in mammals.
In mammalian cells multiple autophagosomes are simultaneously formed adjacently to the ER, in specific regions known as omegasomes, in which DFCP1 concentrates. The activity of the ULK kinase complex, which is constitutively formed by ULK1 or ULK2, ATG13, FIP200 and ATG101, is inhibited for example by mTORC1 in nutrient-rich conditions through the phosphorylation of ULK1 and ATG13. Nutrient deprivation leads to the inhibition of mTORC1 and the concomitant dephosphorylation of ULK1 and ATG13. Active ULK1 kinase complex associates to the recycling endosomes via an unknown mechanism and exits in vesicles that also contain ATG9A and/or the ATG12–ATG5–ATG16L1 complex. ATG9A reaches the recycling endosomes from either the Golgi apparatus or the plasma membrane (PM) by vesicular traffic, while ATG16L1 only from the PM. Recycling endosome-derived vesicles relocalize in the proximity of the ER, possibly through the binding of ULK1 and FIP200 with the ER transmembrane proteins VAPA and VAPB or STX17. The generation of the phagophore very likely involves the fusion of vesicles from different origins, including ATG9A-containing vesicles generated at the Golgi apparatus, COPII-coated vesicles derived from the ERGIC and the HyPAS. The HyPAS is a compartment formed through the fusion between ATG16L1-containing endosomal membranes and cis-Golgi-derived FIP200-positive vesicles, which is mediated by STX17 and its interactors SERCA2, E-SYT2 and SIGMAR1. The lipidation of LC3 already begins on these COPII-coated vesicles and relies on PtdIns3P, which is produced by the PI3K complex I. As a result, transport via COPII-coated vesicles is a possible mechanism for the localization of the PI3K complex I to the nascent phagophore. PtdIns3P is involved in the assembly of the complexes formed by ATG2 and WIPI proteins. The complexes formed by the ATG2 and WIPI proteins probably transfer lipids from the ER to the phagophore. Synthesis of PtdIns3P on the phagophore also leads to the association of ER-localized and PtdIns3P-binding DFCP1, which permits the generation of the characteristic omegasomes. Contrary to yeast, the distribution of the ATG proteins on the phagophore in mammalian cells is unknown and therefore what drawn in the figure is a speculative representation.