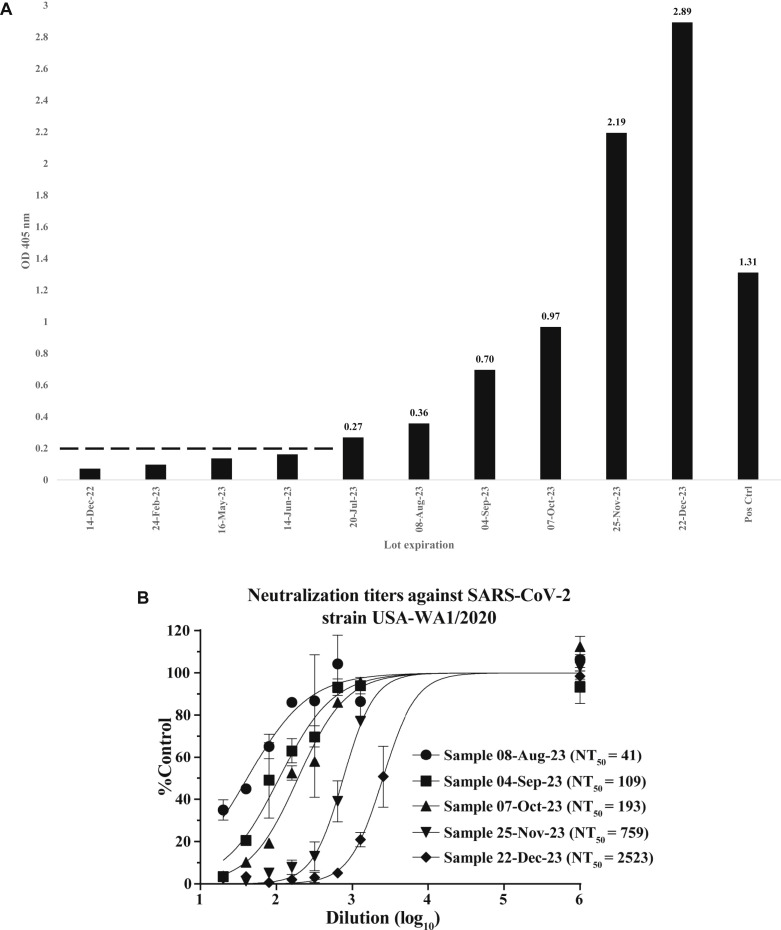
Fig 1.
SARS-CoV-2 antibody assessment of Hizentra lots. A, Ten temporal lots of Hizentra were diluted for detection within the linear range of a SARS-CoV-2–specific ELISA. Lot 1 (expiration date December 14, 2022) is the leftmost sample, and lot 10 (expiration date December 22, 2023) is the rightmost sample, followed by the positive control (de-identified SARS-CoV-2 convalescent patient serum). The results for the 1:1600 dilution are depicted, indicating relative antibody levels (OD405 nm) at lot expiration dates. The threshold for ELISA positivity is shown as a dashed line (OD405 nm ≥ 0.2) established by a 1:400 dilution (not shown). B, All 10 sample lots (lots 6 through 10 are shown) were measured for neutralizing activities against SARS-CoV-2 engineered with an mNeonGreen (mNG) fluorescent reporter virus on VeroE6 cells by fluorescent focus reduction NT (FFRNT) as described previously.3,4 Error bars indicate SDs from duplicates. The nonlinear regression curves of the relative infectivity versus the Hizentra dilutions (log10 values) were created with Prism 9 (GraphPad Software, San Diego, Calif) and used to determine the fold dilution that neutralized 50% of mNG SARS-CoV-2 infectivity (defined as FFRNT50). The calculated NT50 values are shown for sample lots 6 to 10 (expiration dates Aug 8 through December 22, 2023).