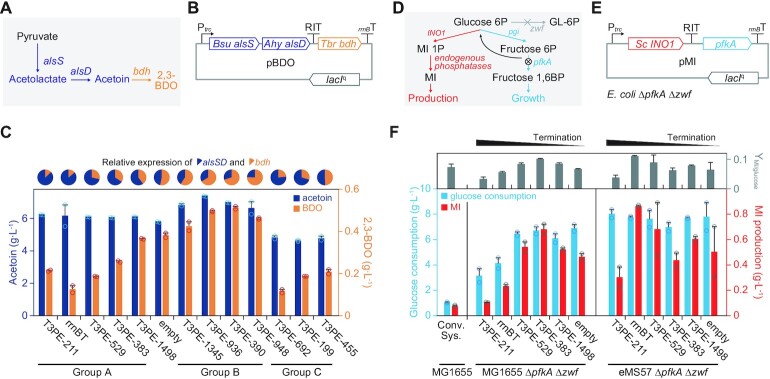
Figure 5.
Stable terminator bioparts can control metabolic pathways in a reliable and scalable manner. (A) Heterologous pathways for acetoin and 2,3-butanediol (2,3-BDO) biosynthesis from pyruvate. Abbreviations are as follows: alsS, acetolactate synthase; alsD, acetolactate decarboxylase; and bdh, butanediol dehydrogenase. (B) The expression of bdh is dependent on the read-through of RITs located upstream. (C) Altered production of acetoin and 2,3-BDO in response to the RIT strengths. Titers of extracellular metabolites were measured after 24 h batch fermentation. Error bars indicate the standard deviations of the two biological replicate cultures. Pie charts indicate the relative expression of alsSD and bdh. (D) Metabolic flux valve system controlling the glycolytic flux for cellular resources and myo-inositol (MI) production. The expression of pfkA involves the metabolic valve of stable terminator bioparts. Abbreviations are as follows: zwf, glucose-6-phosphate dehydrogenase; pgi, glucose-6-phosphatate isomerase; pfkA, 6-phosphofructokinase 1; and INO1, inositol-3-phosphate synthase. (E) Design of the flux-valved MI production pathway. The E. coli ΔpfkA Δzwf strain was used for maximum production and control efficiency. (F) Glucose consumption, MI production, and MI yield from the glucose (g/g) of E. coli harboring one of the pMI plasmids. The conv. sys. indicates the E. coli MG1655 wild-type (both pfkA and zwf were not perturbed) strain heterologously expressing INO1 without a metabolic valve. Error bars indicate the standard deviations of the two biological replicate cultures.