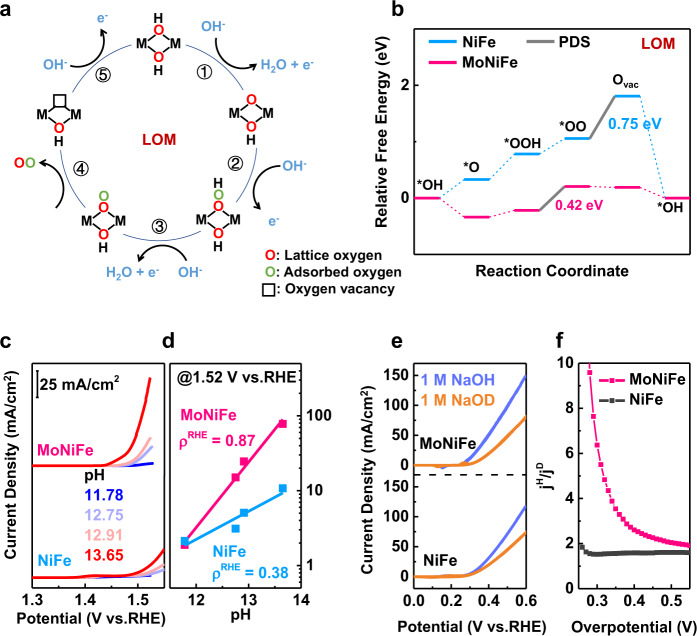
Fig. 4. OER mechanism revealed by DFT calculation, pH dependence, and deuterium isotopic labeling experiments.
a Schematic illustration and b Gibbs free energy diagrams of the LOM pathway on NiFe and MoNiFe (oxy)hydroxide. c Linear sweep voltammetry (LSV) curves for NiFe and MoNiFe (oxy)hydroxide measured in KOH with pH = 11.78, 12.75, 12.91, and 13.65. d OER current density at 1.52 V versus RHE plotted in log scale as a function of pH, from which the proton reaction orders (ρRHE = ∂logi/∂pH) were calculated. e LSV curves for NiFe and MoNiFe (oxy)hydroxide measured in 1 M NaOH and 1 M NaOD solution. The LSV curves are without iR compensation. f The kinetic isotope effect of MoNiFe and NiFe (oxy)hydroxide. jH and jD are referred to the current density measured in NaOH and NaOD solution, respectively.