Abstract
Background
Liver regeneration is a fundamental process for sustained body homeostasis and liver function recovery after injury. Emerging evidence demonstrates that myeloid cells play a critical role in liver regeneration by secreting cytokines and growth factors. Peroxisome proliferator-activated receptor α (PPARα), the target of clinical lipid-lowering fibrate drugs, regulates cell metabolism, proliferation, and survival. However, the role of myeloid PPARα in partial hepatectomy (PHx)-induced liver regeneration remains unknown.
Methods
Myeloid-specific PPARa-deficient (PparaMye−/−) mice and the littermate controls (Pparafl/fl) were subjected to sham or 2/3 PHx to induce liver regeneration. Hepatocyte proliferation and mitosis were assessed by immunohistochemical (IHC) staining for 5-bromo-2'-deoxyuridine (BrdU) and Ki67 as well as hematoxylin and eosin (H&E) staining. Macrophage and neutrophil infiltration into livers were reflected by IHC staining for galectin-3 and myeloperoxidase (MPO) as well as flow cytometry analysis. Macrophage migration ability was evaluated by transwell assay. The mRNA levels for cell cycle or inflammation-related genes were measured by quantitative real-time RT-PCR (qPCR). The protein levels of cell proliferation related protein and phosphorylated signal transducer and activator of transcription 3 (STAT3) were detected by Western blotting.
Results
PparaMye−/− mice showed enhanced hepatocyte proliferation and mitosis at 32 h after PHx compared with Pparafl/fl mice, which was consistent with increased proliferating cell nuclear antigen (Pcna) mRNA and cyclinD1 (CYCD1) protein levels in PparaMye−/− mice at 32 h after PHx, indicating an accelerated liver regeneration in PparaMye−/− mice. IHC staining showed that macrophages and neutrophils were increased in PparaMye−/− liver at 32 h after PHx. Livers of PparaMye−/− mice also showed an enhanced infiltration of M1 macrophages at 32 h after PHx. In vitro, Ppara-deficient bone marrow-derived macrophages (BMDMs) exhibited markedly enhanced migratory capacity and upregulated M1 genes Il6 and Tnfa but downregulated M2 gene Arg1 expressions. Furthermore, the phosphorylation of STAT3, a key transcript factor mediating IL6-promoted hepatocyte survival and proliferation, was reinforced in the liver of PparaMye−/− mice after PHx.
Conclusions
This study provides evidence that myeloid PPARα deficiency accelerates PHx-induced liver regeneration via macrophage polarization and consequent IL-6/STAT3 activation, thus providing a potential target for manipulating liver regeneration.
Keywords: Liver regeneration, peroxisome proliferator-activated receptor α (PPARα), myeloid cell, signal transducer and activator of transcription 3 (STAT3), interleukin 6 (IL-6)
Introduction
Liver is the only organ that maintains a remarkable capacity to regenerate through hepatocellular hypertrophy and hyperplasia, which is described in experimental models, such as two-thirds partial hepatectomy (PHx) in rodents (1).The regenerative capability protects the organism from parenchyma loss, which may be caused by hepatotoxins, chronic infection, PHx, and liver transplantation. Impaired regeneration exacerbates liver dysfunction during the above processes. Understanding the hepatic regenerative process is of great clinical significance as the effectiveness of many treatments for chronic liver diseases, such as donor liver transplantation and tumor resections, depends on efficient liver regeneration. Accordingly, it is necessary to explore new therapeutic targets for liver regeneration.
Liver regeneration is a tightly controlled and compensatory process, in which multiple cell types and signaling molecules are involved. For instance, early after PHx, increased secretion and utility of growth factors [hepatocyte growth factor (HGF), epidermal growth factor (EGF)], hormones (norepinephrine, insulin, serotonin), and cytokines [interleukin 6 (IL-6), tumor necrosis factor α (TNF-α)] are initiated and consequently prime the activation of transcription factors [signal transducer and activator of transcription (STAT) 3; nuclear factor κB (NF-κB); β-catenin]. These early events guide the changes in transcriptome and the following cell cycle progression of quiescent hepatocytes. Subsequently, the parenchymal and nonparenchymal cells proliferate until the liver mass is restored (2-5). However, the source of the cytokines and growth factors have not been fully explored.
Abundance experimental evidence points that the innate and adaptive immune cells are necessary for normal liver regeneration after PHx (6-8). After acute liver injury, damaged hepatocyte releases danger-associated molecular patterns (DAMPs) activate Kupffer cells and hepatic stellate cells, resulting in the release of chemokines, such as CCL2 and IL-8 (CXCL8), that recruit myeloid cells into local areas of inflammation (9). Gut-derived factors, such as lipopolysaccharide (LPS), activate hepatic Kupffer cells and promote them to produce TNF-α and IL-6. IL-6 binds to its receptor on hepatocytes, triggers activation of the Janus kinase (JAK)-STAT3 pathway, and ultimately promotes hepatocyte survival and proliferation (2,4,6,10). Hitherto the transcription factors regulating myeloid cell function during PHx are not completely understood.
Nuclear receptor peroxisome proliferator-activated receptor α (PPARα) is the target of widely-used hypolipidemic fibrate drugs mainly via control of fatty acid metabolism (11). Short-term PPARα activation by its synthetic agonist fibrates and Wy-14643 induces hepatomegaly, and long-term activation causes hepatocarcinogenesis in a PPARα-dependent manner in rodents (12-15). Whole-body knockout of PPARα impairs PHx-induced liver regeneration (16,17), whereas hepatocyte-specific PPARα deficiency has a less extent of impairment in PHx-induced liver regeneration than the PPARα whole-body knockout (18), indicating that non-parenchymal PPARα may also play a certain role in PHx-induced liver regeneration. Recent studies suggested that PPARα activation is critical for the anti-inflammatory effects of myeloid cells (19,20). However, the role of myeloid PPARα in PHx-induced liver regeneration remains unknown.
In this study, the role of myeloid PPARα in PHx-induced liver regeneration was explored by genetic manipulation of PPARα in myeloid cells. Myeloid PPARα-deficient (PparaMye−/−) mice exhibited accelerated liver regeneration after PHx. Mechanistically, PPARα deficiency increased IL-6 expression in myeloid cells via promoting M1 polarization and consequently activated STAT3 in the liver, thus providing a potential target for manipulating liver regenerative disease. We present the following article in accordance with the ARRIVE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-688/rc).
Methods
Animal experiments
C57BL/6J wild-type mice were purchased from Charlies River Company (Beijing, China). Pparafl/fl and myeloid-specific Ppara-deficient (Pparafl/fl;LysM-Cre, PparaMye−/−) mice were generated as described previously (20). All mice were housed and bred in the animal facilities at Capital Medical University according to Chinese guidelines. All animals were maintained on a 12-h light/12-h dark cycle and free access to food and water. Animal experiments were performed under a project license (AEEI-2018-127) granted by ethics board of Capital Medical University.
Eight- to 10-week-old male Pparafl/fl and PparaMye−/− mice were used in this study. Two-thirds PHx-induced liver regeneration models were performed as previously described (21,22). Mice were injected with 5-bromo-2'-deoxyuridine (BrdU, 50 mg/kg body weight) 2 h before sacrificing at the indicated time points. Liver tissues were fixed in formalin, embedded in paraffin, or frozen in optimal cutting temperature compound (OCT) for cryosection, whereas the remaining liver tissue was snap-frozen for further analysis.
Hematoxylin and eosin (H&E) staining and immunohistochemistry staining
Paraffin-embedded liver tissues were cut into sections (4-µm thick) for H&E and immunohistochemistry staining. H&E staining was performed following standard methods. Immunohistochemistry analysis was performed using antibodies against galectin-3 (Santa Cruz Biotechnology, California, USA), myeloperoxidase (MPO) (Abcam, Cambridge, UK) and Ki67 [Cell Signaling Technology (CST), Boston, USA] following standard instructions and antibodies against BrdU (BD Bioscience, San Jose, CA, USA), as previously described (23).
Liver macrophage isolation
Mouse liver macrophages were separated by gradient centrifugation as previous described (24). Briefly, mouse liver was perfused and digested with collagenase solution. Liver was further digested with Dulbecco’s Modified Eagle’s Medium (DMEM, Corning, New York, USA) containing 1% collagenase Ⅳ in a 37 ℃ water bath for 30 min in vitro. After filtering the liver cell suspension, cells were centrifuged twice at 50 g for 2 min to remove hepatocytes. Hepatic nonparenchymal cells were further centrifuged at 400 g for 10 min. Cell pellets were resuspended with Percoll gradient (25%+50%) solution and centrifuged at 1,600 g for 17 min without a break. macrophage layers (between 25% and 50% Percoll gradient) were collected, washed with PBS, and resuspended in DMEM containing 10% fetal bovine serum (FBS) and 100 U/mL of penicillin/streptomycin. Cells were cultured in a six‐well plate at 37 ℃. After 4 h, nonadherent cells were removed by aspiration and macrophages were washed with PBS 3 times.
Bone marrow-derived macrophages (BMDMs) isolation and treatment
Bone marrow-derived cells were isolated from the femurs and tibias of adult Pparafl/fl and PparaMye−/− mice as previously described (25). Cells were planted in DMEM complete medium (10% FBS and 100 U/mL of penicillin/streptomycin) and stimulated with murine macrophage colony stimulating factor (50 ng/mL) for 3 days to allow the differentiation into macrophages. For cell experiment, the BMDMs were stimulated with vehicle and LPS (100 ng/mL) for 3 h to simulate inflammation response.
Flow cytometry
Liver non-parenchymal cells were isolated and single-cell suspensions were treated with Fc block, washed, and stained with CD45 percpCy5.5 (557235, BD, USA), CD11b FITC (557396, BD, USA), F4/80 BV421 (565411, BD, USA), Ly6G APC (560599, BD, USA), CD206 PE (141706, BD, USA), and their homologous isotype-matched negative controls (BD, Franklin Lakes, NJ, USA). In the basis of a live gate, events were acquired on a Fortessa flow cytometer (BD, USA) and analyzed by FlowJo V10 software (BD, USA).
Transwell migration assay
BMDMs were seeded at a density of 2×106 cells/mL in a 5-μm pore-size transwell chamber (Corning, New York, USA) with DMEM containing 1% FBS and 100 U/mL of penicillin/streptomycin. DMEM containing 10% FBS were added in the bottom of each well as a chemoattractant. After incubation at 37 ℃ for 24 h, chambers were removed and washed by PBS, fixed with 4% paraformaldehyde for 20 min, stained with 0.25% crystal violet (DZ0059, Leagene, Beijing, China) for 20 min and cells in the upper chamber were depleted. The average value of migrated cells was counted in five fields per membrane to determine the migration ability.
RNA extraction and real-time quantitative PCR
Total RNA was extracted from the liver tissues, BMDMs, or liver macrophages of Pparafl/fl and PparaMye−/− mice using TRIzol reagent (Life Technologies, Carlsbad, CA, USA). One μg of total RNA was reverse-transcribed into cDNA with GoScript Reverse Transcriptase (Promega, Madison, USA) and subjected to quantitative real-time RT-PCR (qPCR) analysis with SYBR Green premix (TaKaRa, Nojihigashi, Kusatsu, Shiga, Japan) on CFX Connect Real-Time System (Bio-Rad, Hercules, CA, USA). Relative expression of target genes was calculated by 2−ΔΔCt method and normalized to that of the housekeeping gene Actb mRNA. The primers are listed in Table S1.
Western blot
Whole-cell lysate was extracted using tissue protein extraction reagent (Thermo Scientific, Waltham, MA, USA). The protein concentration was measured using the bicinchoninic acid protein assay kit (Thermo Scientific). Specific primary antibodies used were as follows: antibodies against pSTAT3 (CST, Boston, USA), STAT3α (CST, Boston, USA), pSTAT1 (CST, Boston, USA), STAT1 (CST, Boston, USA), ACTB (Proteintech, Chicago, USA). The dilutions were 1:1,000 in 5% bovine serum albumin. After incubating with horseradish peroxidase-conjugated secondary antibody (MilliporeSigma, Darmstadt, Germany), the immunocomplexes were visualized with FluorChem-R (ProteinSimple, San Jose, CA, USA). Total protein levels were normalized to ACTB.
Statistical analysis
The mean ± standard deviation (SD) was calculated and plotted using GraphPad Prism 7 software (GraphPad Software, San Diego, California, USA). Comparisons between two groups were performed using two-tailed unpaired Student’s t-test. Differences between multiple groups with one variable were determined using one-way analysis of variance (one-way ANOVA) followed by Bonferroni’s post-hoc test. To compare multiple groups with more than one variable, two-way ANOVA followed by Bonferroni’s post-hoc test was used. P<0.05 was considered statistically significant.
Results
Deficiency of myeloid PPARα accelerates PHx-induced liver regeneration
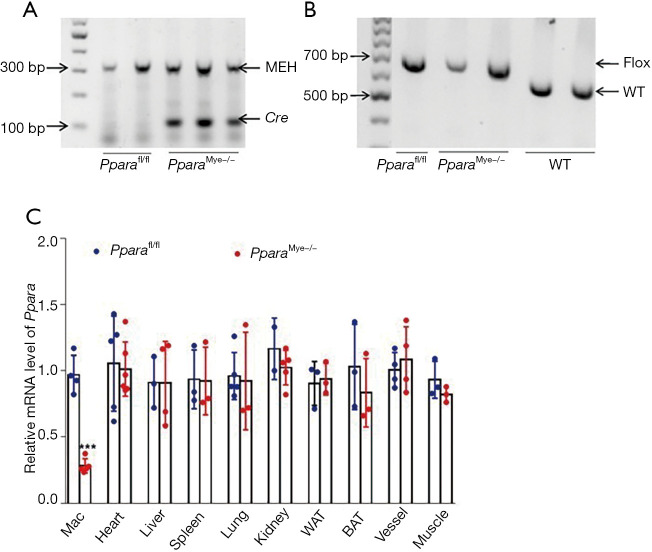
To explore the role of myeloid PPARα in PHx-induced hepatocyte proliferation, Pparafl/fl mice were crossed with transgenic mice expressing Cre recombinase under control of lysozyme 2 (LysMCre) promoters to generate myeloid-specific Ppara knockout (PparaMye−/−) mice as previous described (20). PCR analyses demonstrated Cre allele and Ppara homogenous alleles in PparaMye−/− genomic DNA (Figure 1A,1B). qPCR analysis showed that Ppara mRNA level was specifically decreased in BMDMs from PparaMye−/− mice but not from Pparafl/fl mice (Figure 1C), indicating a successful construction of myeloid-specific Ppara-deficient mice.
Figure 1.
Identification of myeloid PPARα-deficient mice. (A,B) PCR analyses for Cre and Ppara allele in genomic DNA from Pparafl/fl and PparaMye−/− mice. (C) qPCR analysis for Ppara mRNA levels in macrophages or tissues from Pparafl/fl and PparaMye−/− mice (n=3–5). Data are expressed as means ± SD, n=3–5. ***, P<0.001. MEH, musculus epoxide hydrolase 1; WT, wild type; Mac, macrophage; WAT, white adipose tissue; BAT, brown adipose tissue; qPCR, quantitative real-time RT-PCR.
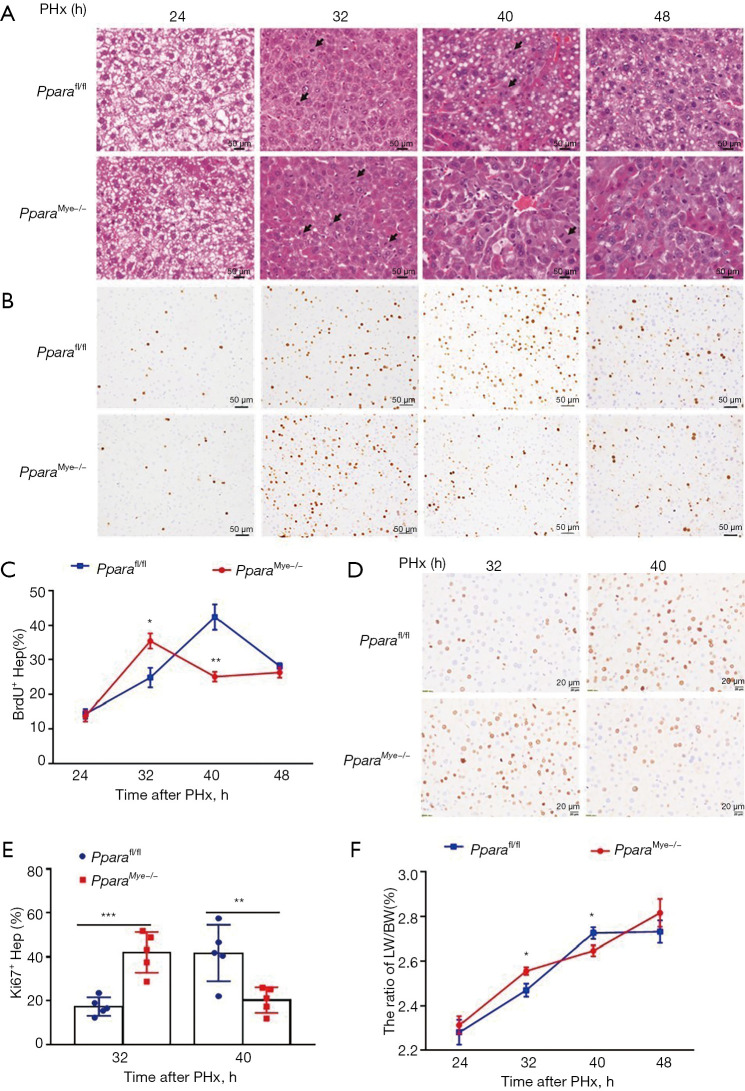
The Pparafl/fl and PparaMye−/− mice were subjected to 2/3 PHx operation. H&E staining showed a raised number of mitotic cells in PparaMye−/− liver at 32 h (Figure 2A). Immunohistochemical staining demonstrated an increase of BrdU+ (Figure 2B,2C) and Ki67+ (Figure 2D,2E) hepatocytes at 32 h but dropdown at 40 h after PHx in PparaMye−/− mice compared with Pparafl/fl mice. Consistently, the liver-to-body weight of PparaMye−/− mice was increased at 32 h than that of Pparafl/fl mice (Figure 2F). Taken together, these results indicate an accelerated liver regeneration in myeloid PPARα-deficient mice after PHx.
Figure 2.
Myeloid PPARα deficiency mice show accelerated liver regeneration after PHx. (A) Representative H&E staining for Pparafl/fl and PparaMye−/− livers at 24, 32, 40, and 48 h after PHx. The arrows refer to mitotic hepatocytes. (B) Representative IHC staining for BrdU in Pparafl/fl and PparaMye−/− livers at 24, 32, 40, and 48 h after PHx. (C) BrdU+ hepatocyte/total hepatocyte ratios at indicated time points. (D) Representative IHC staining for Ki67 in Pparafl/fl and PparaMye−/− livers at 32 and 40 h after PHx. (E) Ki67+ hepatocyte/total hepatocyte ratios at indicated time points. (F) The ratio of LW/BW at indicated time points. Data are expressed as means ± SD, n=5–11. *, P<0.05; **, P<0.01; ***, P<0.001. PHx, partial hepatectomy; LW/BW, liver weight/body weight.
PPARα disruption in myeloid cells promotes cell cycle progression after PHx
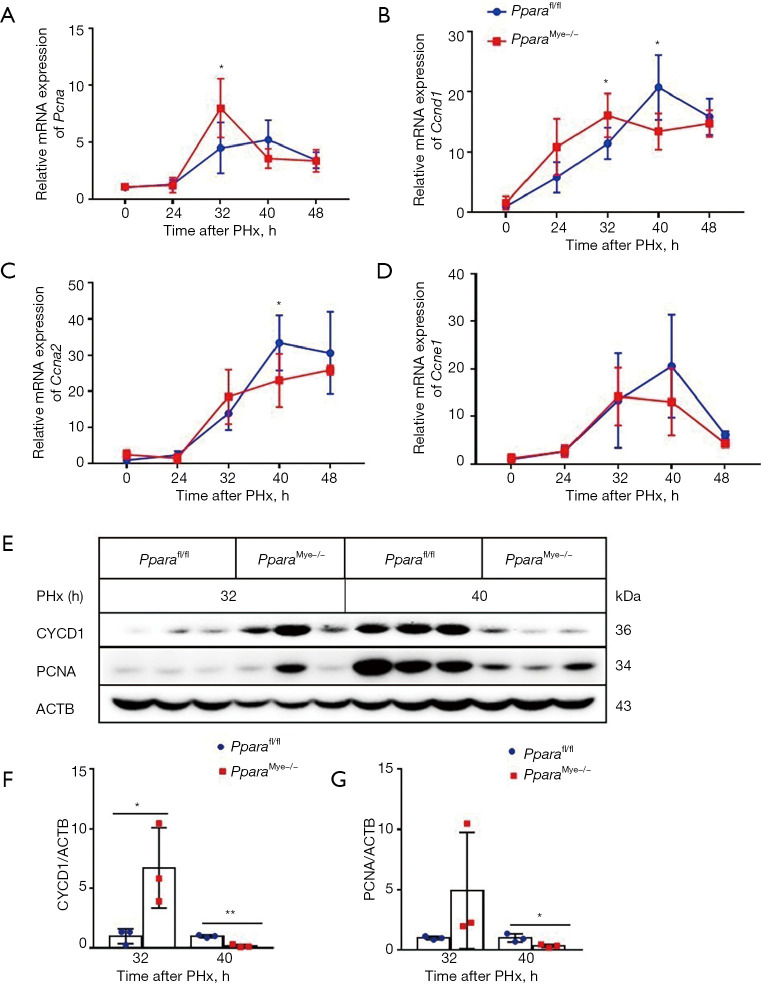
To further confirm whether myeloid PPARα deficiency accelerates PHx-induced liver regeneration, the cell cycle-related genes, such as Pcna, Ccnd1, Ccna2 and Ccne1 were measured by qPCR analysis. As shown in Figure 3, Ccnd1 and Pcna mRNA were increased at 32 h after PHx (Figure 3A,3B) in PparaMye−/− mouse liver compared to Pparafl/fl mice, indicating an advanced G1–S progression after myeloid PPARα deficiency. However, Ccna2 and Ccne1 expression was comparable between these two groups (Figure 3C,3D). Western blot analyses also confirmed an enhanced CYCD1 expression at 32 h after PHx in PparaMye−/− mice compared to Pparafl/fl mice (Figure 3E,3F). However, the PCNA protein expression was comparable in Pparafl/fl and PparaMye−/− mice at 32 h after PHx (Figure 3E,3G). It is reported that mitogenic growth factors HGF and EGF involved in regulating liver regeneration (26). Therefore, the expression Hgf and Egf was measured, no difference was found between Pparafl/fl and PparaMye−/− mice at 32 h after PHx (data not shown). These results indicating that Hgf and Egf may not be the main genes regulated by myeloid PPARα in liver regeneration. These results confirm an accelerated G1–S progression in PparaMye−/− mice after PHx.
Figure 3.
Myeloid PPARα deficiency mice show increased cell cycle-related gene expression at 32 h after PHx. (A-D) qPCR analysis for mRNA levels of cell cycle related gene Pcna, Ccnd1, Ccna2, and Ccne1 in Pparafl/fl and PparaMye−/− liver at 0, 24, 32, 40, and 48 h after PHx (n=5). (E-G) Western blot analysis of CYCD1 and PCNA protein levels in Pparafl/fl and PparaMye−/− liver at 32 and 40 h after PHx (n=3). Data are expressed as means ± SD. *, P<0.05; **, P<0.01. PHx, partial hepatectomy; qPCR, quantitative real-time RT-PCR.
Myeloid PPARα deficiency promotes M1 phenotype macrophage infiltration to the liver at 32 h after PHx
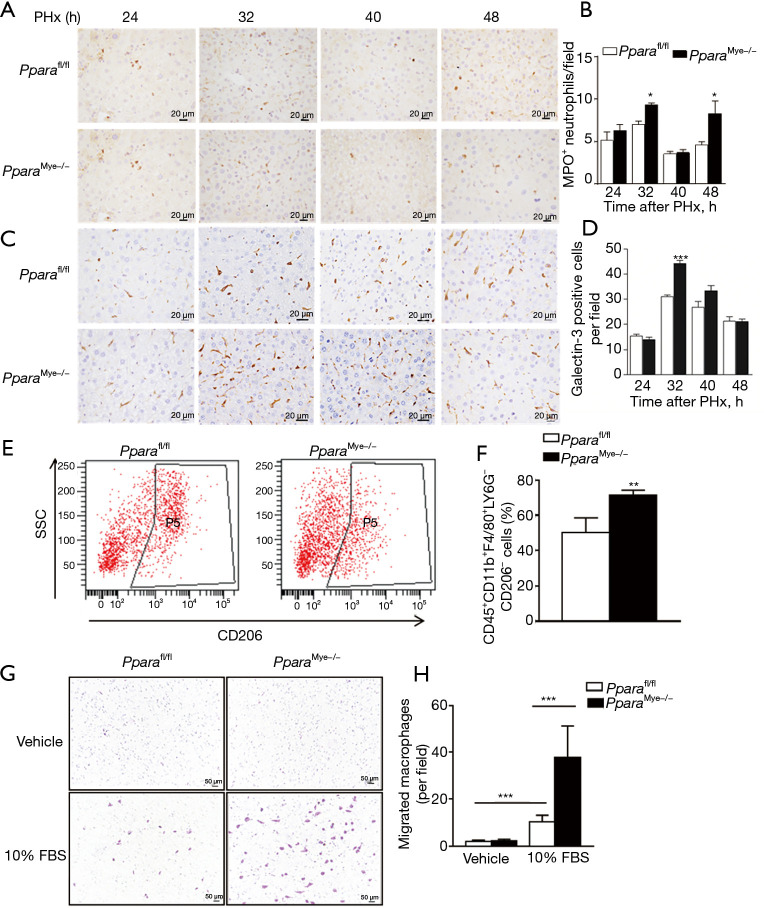
It is well established that inflammatory cells infiltrate into the liver after acute PHx operation and promotes liver regeneration (7). To investigate the influence of myeloid PPARα in inflammatory cells infiltration to liver, immunohistochemical staining for MPO (Figure 4A,4B) and galectin-3 (Figure 4C,4D) was performed. There was a marked increase of neutrophil and macrophage infiltration to the liver at 32 h after PHx in PparaMye−/− mice compared with Pparafl/fl mice. To deeply understanding the relationship between myeloid PPARα and liver regeneration, the flow cytometry assay was performed. PPARα deficiency in myeloid cells dramatically increased the infiltration of M1 (CD45+CD11b+F4/80+LY6G−CD206−) macrophages at 32 h after PHx (Figure 4E,4F). In vitro, the Ppara-deficient BMDMs exhibited markedly enhanced migratory capacity as reflected by modified Boyden chamber assay (Figure 4G,4H). Taken together, myeloid PPARα deficiency promotes liver macrophage infiltration by promoting macrophage migration at 32 h after PHx.
Figure 4.
Myeloid PPARα deficiency promotes M1 phenotype macrophages infiltration to the liver at 32 h after PHx. (A,B) Representative IHC staining for MPO in Pparafl/fl and PparaMye−/− livers at 24, 32, 40, and 48 h after PHx (A) and quantification of MPO+ cells (B) (n=5). (C,D) Representative IHC staining for galectin-3 in Pparafl/fl and PparaMye−/− livers at 24, 32, 40, and 48 h after PHx (C) and quantification of galectin-3+ cells (D) (n=5). (E,F) Flow cytometry analysis of CD45+CD11b+F4/80+LY6G−CD206−cell subtype in the Pparafl/fl and PparaMye−/− livers at 32 h after PHx and quantification (n=4). (G,H) Representative images of macrophage migration upon vehicle or 10% FBS stimulation by transwell assay (G) and quantification (H) (n=6). Data are expressed as means ± SD. *, P<0.05; **, P<0.01; ***, P<0.001. PHx, partial hepatectomy; MPO, myeloperoxidase; SSC, side scatter.
Myeloid PPARα deficiency enhances BMDMs polarization to M1 phenotype
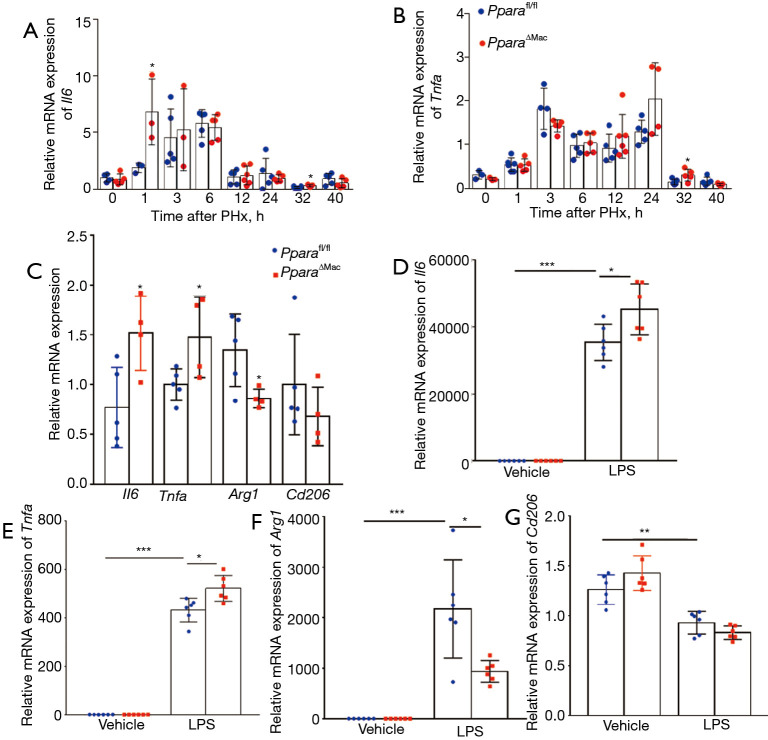
IL-6 pathways are essential for most of the immediate early gene expression to lead quiescent hepatocytes into a proliferative state after PHx operation (27-29) and M1 macrophages secreting IL-6 and TNF-α (30). To further understating the role of myeloid PPARα in macrophage polarization and liver regeneration, the mRNA level of Il6 and Tnfa in liver tissues was measured. qPCR analysis showed enhanced expression of Il6 and Tnfa at 32 h in livers of PparaMye−/− mice after PHx compared with Pparafl/flmice (Figure 5A,5B). In vitro, the liver macrophages were isolated to measuring macrophage polarization related gene expression. The results showed increased M1 macrophage marker Il6, Tnfa expression and decreased M2 macrophage marker, Arg1, expression in liver macrophages at 32 h after PHx (Figure 5C). To further explore the role of myeloid PPARα in macrophage polarization, the BMDMs were treated with vehicle or LPS for 3 h, as PHx leads to elevation of serum levels of LPS, qPCR analysis showed increased M1 macrophage marker Il6, Tnfa expression and decreased M2 macrophage marker, Arg1, expression in BMDMs of PparaMye−/− mice (Figure 5D-5F). However, no difference was found in Cd206 expression of BMDMs from Pparafl/fl and PparaMye−/− mice after LPS stimulation (Figure 5G). In all, these results indicate that myeloid PPARα promotes macrophage polarization into pro-inflammatory M1 phenotype, thus accelerates the process of liver regeneration.
Figure 5.
Myeloid PPARα deficiency enhances BMDMs polarization to M1 phenotype. (A,B) qPCR analysis of Il6 and Tnfa mRNA expression in livers of Pparafl/fl and PparaMye−/− mice at indicated time points (n=3–6). (C) qPCR analysis of Il6, Tnfa, Arg1 and Cd206 mRNA levels in liver macrophages of Pparafl/fl and PparaMye−/− mice at 32 h after PHx (n=4–5). (D-G) qPCR analysis of Il6, Tnfa, Arg1 and Cd206 mRNA levels in BMDMs of Pparafl/fl and PparaMye−/− mice treated with vehicle or LPS (100 ng/mL) for 3 h (n=6). Data are expressed as means ± SD. *, P<0.05; **, P<0.01; ***, P<0.001. BMDMs, bone marrow-derived macrophages; PHx, partial hepatectomy; LPS, lipopolysaccharide.
Myeloid PPARα deficiency increases STAT3 phosphorylation in liver at 32 h after PHx
It is widely known that STAT3, a key transcript factor, mediating the effect of IL-6 on hepatocyte survival and proliferation after PHx (6), while STAT1 activation plays a role in inhibiting liver regeneration (31,32). To exploring the molecular mechanism for the accelerated liver regeneration in PparaMye−/− mice, STAT3 and STAT1 phosphorylation was assessed by Western blot. An increase of phosphorylated STAT3 in PparaMye−/− mice was observed at 32 h after PHx, although phosphorylation of STAT3 was decreased at 40 h after PHx (Figure 6A,6B), which was consistent with accelerated hepatocyte proliferation. However, no difference of phosphorylation of STAT1 expression was found between Pparafl/fl and PparaMye−/− liver at 32 or 40 h after PHx (Figure 6A,6C). These results suggesting that myeloid PPARα may delay liver regeneration via IL-6/STAT3 signaling.
Figure 6.
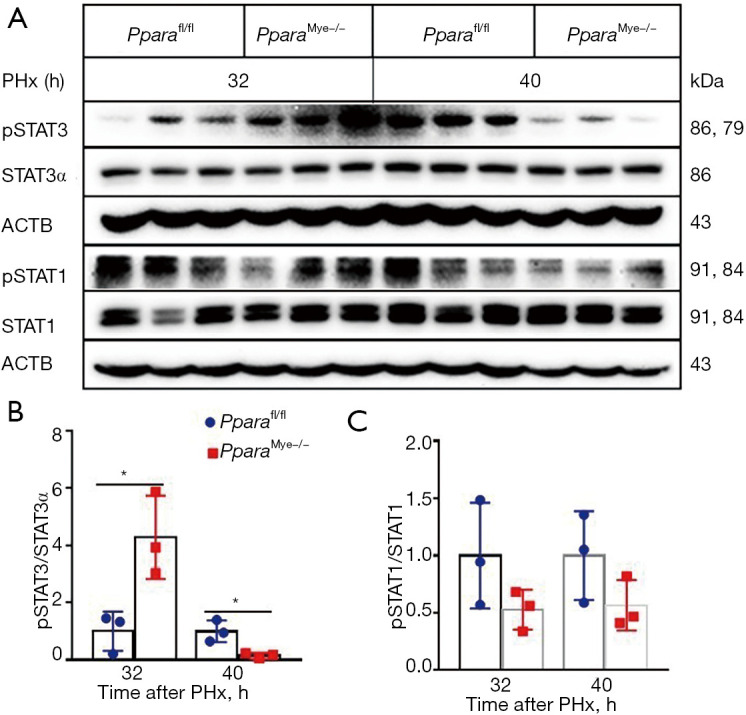
Myeloid PPARα deficiency increases STAT3 phosphorylation in liver at 32 h after partial hepatectomy (PHx). (A) Western blot analysis of phosphorylated and total protein of STAT3, and STAT1 in Pparafl/fl and PparaMye−/− livers at 32 and 40 h after PHx. (B,C) Quantification of phosphorylated STAT3 (B) and STAT1 (C). Data are expressed as means ± SD, n=3. *, P<0.05.
Discussion
In the current study, myeloid disruption of PPARα led to accelerated liver regeneration after PHx. In addition, myeloid PPARα deficiency markedly increased intrahepatic neutrophils and macrophages, especially M1 macrophages infiltration, as well as the expression of inflammatory factors, such as IL-6 and TNF-α, which are essential for efficient liver regeneration (33,34). As a result, STAT3, a central component in the inflammatory signaling cascade mediating the mitogenic responses of hepatocytes to inflammatory factors after PHx (6,35), was significantly activated. Accordingly, enhanced cyclin D1 induction in regenerating livers of PparaMye−/− mice was observed. These results demonstrate that liver regeneration is modulated by intrahepatic immune microenvironment but not hepatic parenchymal cells alone.
PPARα is a ligand-inducible nuclear receptor of clinical interest as fibrate drug target via controlling fatty acid metabolism in various metabolic disorders (36). Short-term PPARα agonist treatment was shown to induce hepatocyte proliferation and hepatomegaly in rodents (15,37,38), which is mainly hepatocyte PPARα dependent, as either whole-body or hepatocyte-specific PPARα knockout completely abolished this hepatic proliferation (18,20). Sustained PPARα activation contributed to hepatocarcinoma in rodents (13,14,39). It was proposed that the underlying mechanism is PPARα-dependent downregulation of microRNA Let7c, which in turn released the repression of oncogene c-Myc (40,41). All the above findings emphasized a critical role of PPARα in peroxisome proliferator-induced hepatocyte proliferation. In the context of PHx, whole-body PPARα-deficient mice exhibited a significant impairment of liver regeneration, which was associated with altered expression of genes involved in cell cycle control, cytokine signaling, and fat metabolism (16). However, compared to whole-body PPARα-knockout mice, hepatocyte-specific PPARα-deficient mice showed a less extent of delay in PHx-induced liver regeneration by inhibiting cell cycle progression and lipid metabolism, indicating an intrinsic compensative or restrictive mechanism for PPARα from other cell types. In this study, a significant increase of hepatocyte proliferation as well as cell cycle gene Ccnd1 mRNA and protein levels was observed in PparaMye−/− mice at 32 h post PHx, which suggests that myeloid PPARα deficiency accelerates PHx-induced liver regeneration, thus indicating that myeloid PPARα might serve as an endogenous restrictive mechanism for the proliferative effects of hepatocyte PPARα activation.
Emerging evidence support the crucial role of myeloid cells in PHx-induced liver regeneration (4,42-44). Myeloid cells secret proinflammatory cytokines, such as IL-6 and TNF-α, which are known to activate quiescent hepatocytes enter cell cycle (29). In this study, myeloid PPARα deficiency significantly increased the infiltration of macrophages and neutrophils into the livers, especially M1 macrophages, which is consistent with the anti-inflammatory properties of PPARα in macrophages and other cell types, such as endothelial cells (11,45). In addition, chemotaxis mediators, such as osteopontin, monocyte chemoattractant protein 1 (MCP-1), and intercellular adhesion molecule-1 (ICAM-1) recruit macrophages to liver tissues after PHx (10,46-48), myeloid PPARα deficiency significantly enhanced the migratory compacity at 32 h after PHx, which may account for increased macrophage infiltration to liver tissues. Although further studies are still needed for the precise mechanism for how PPARα activation yields an anti-inflammatory profile, a possible hypothesis is that PPARα negatively inferences with proinflammatory transcription factors, such as NF-κB and AP1 (11).
Genetic manipulation or pharmacological inhibitors illustrate that IL-6/STAT3 pathway is necessary for the initiation of hepatocyte mitogenesis and proliferation (23), while STAT1 activation plays a role in inhibiting liver regeneration (31,32). As a downstream of IL-6, STAT3 also play an anti-inflammatory role in macrophage (49). On the contrary, STAT1 serve as a pro-inflammatory transcription factors in macrophage (49). In this study, myeloid PPARα deficiency enhanced the phosphorylation of STAT3 in liver at 32 h after PHx, but have no influence on the phosphorylation of STAT1. Although STAT3 serve as a marker of M2 macrophage, hepatocytes IL-6/STAT3 pathway activation is very important for efficient liver regeneration after PHx. These results confirmed the key role of IL-6/STAT3 pathway in accelerated liver regeneration in myeloid Ppara-deficient mice.
In conclusion, this study indicates that myeloid PPARα restricts PHx-induced liver regeneration via inhibiting IL-6/STAT3 pathway, thus providing a potential target for manipulating liver regeneration under the conditions of liver injury or liver transplantation.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (81370521, 81670400, and 91739120), National Key R&D Program of China (2017YFC0211600), Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (CIT&TCD20190332), The Key Science and Technology Project of Beijing Municipal Institutions (KZ202010025032).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (AEEI-2018-127) granted by ethics board of Capital Medical University, in compliance with Chinese guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-688/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-688/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-688/coif). The authors have no conflicts of interest to declare.
References
- 1.Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol 2016;13:473-85. 10.1038/nrgastro.2016.97 [DOI] [PubMed] [Google Scholar]
- 2.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 2006;43:S45-53. 10.1002/hep.20969 [DOI] [PubMed] [Google Scholar]
- 3.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology 2009;137:466-81. 10.1053/j.gastro.2009.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017;65:1384-92. 10.1002/hep.28988 [DOI] [PubMed] [Google Scholar]
- 5.Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol 2021;18:40-55. 10.1038/s41575-020-0342-4 [DOI] [PubMed] [Google Scholar]
- 6.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 2004;5:836-47. 10.1038/nrm1489 [DOI] [PubMed] [Google Scholar]
- 7.Li N, Hua J. Immune cells in liver regeneration. Oncotarget 2017;8:3628-39. 10.18632/oncotarget.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin S, Wang H, Bertola A, et al. Activation of invariant natural killer T cells impedes liver regeneration by way of both IFN-gamma- and IL-4-dependent mechanisms. Hepatology 2014;60:1356-66. 10.1002/hep.27128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weston CJ, Zimmermann HW, Adams DH. The Role of Myeloid-Derived Cells in the Progression of Liver Disease. Front Immunol 2019;10:893. 10.3389/fimmu.2019.00893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selzner N, Selzner M, Odermatt B, et al. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology 2003;124:692-700. 10.1053/gast.2003.50098 [DOI] [PubMed] [Google Scholar]
- 11.Bougarne N, Weyers B, Desmet SJ, et al. Molecular Actions of PPARalpha in Lipid Metabolism and Inflammation. Endocr Rev 2018;39:760-802. 10.1210/er.2018-00064 [DOI] [PubMed] [Google Scholar]
- 12.Woods CG, Burns AM, Bradford BU, et al. WY-14,643 induced cell proliferation and oxidative stress in mouse liver are independent of NADPH oxidase. Toxicol Sci 2007;98:366-74. 10.1093/toxsci/kfm104 [DOI] [PubMed] [Google Scholar]
- 13.Hays T, Rusyn I, Burns AM, et al. Role of peroxisome proliferator-activated receptor-alpha (PPARalpha) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis 2005;26:219-27. 10.1093/carcin/bgh285 [DOI] [PubMed] [Google Scholar]
- 14.Klaunig JE, Babich MA, Baetcke KP, et al. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol 2003;33:655-780. 10.1080/713608372 [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Youssef J, Cunningham M, et al. Correlation between thyroid hormone status and hepatic hyperplasia and hypertrophy caused by the peroxisome proliferator-activated receptor alpha agonist Wy-14,643. J Carcinog 2004;3:9. 10.1186/1477-3163-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson SP, Yoon L, Richard EB, et al. Delayed liver regeneration in peroxisome proliferator-activated receptor-alpha-null mice. Hepatology 2002;36:544-54. 10.1053/jhep.2002.35276 [DOI] [PubMed] [Google Scholar]
- 17.Wheeler MD, Smutney OM, Check JF, et al. Impaired Ras membrane association and activation in PPARalpha knockout mice after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 2003;284:G302-12. 10.1152/ajpgi.00175.2002 [DOI] [PubMed] [Google Scholar]
- 18.Xie G, Yin S, Zhang Z, et al. Hepatocyte Peroxisome Proliferator-Activated Receptor alpha Enhances Liver Regeneration after Partial Hepatectomy in Mice. Am J Pathol 2019;189:272-82. 10.1016/j.ajpath.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res 2000;49:497-505. 10.1007/s000110050622 [DOI] [PubMed] [Google Scholar]
- 20.Brocker CN, Yue J, Kim D, et al. Hepatocyte-specific PPARA expression exclusively promotes agonist-induced cell proliferation without influence from nonparenchymal cells. Am J Physiol Gastrointest Liver Physiol 2017;312:G283-99. 10.1152/ajpgi.00205.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Feng D, Park O, et al. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-gamma. Hepatology 2013;58:1474-85. 10.1002/hep.26471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell C, Willenbring H. Addendum: A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc 2014. doi: . 10.1038/nprot.2014.122 [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Park O, Lafdil F, et al. Interplay of hepatic and myeloid signal transducer and activator of transcription 3 in facilitating liver regeneration via tempering innate immunity. Hepatology 2010;51:1354-62. 10.1002/hep.23430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aparicio-Vergara M, Tencerova M, Morgantini C, et al. Isolation of Kupffer Cells and Hepatocytes from a Single Mouse Liver. Methods Mol Biol 2017;1639:161-71. 10.1007/978-1-4939-7163-3_16 [DOI] [PubMed] [Google Scholar]
- 25.Qu A, Shah YM, Manna SK, et al. Disruption of endothelial peroxisome proliferator-activated receptor gamma accelerates diet-induced atherogenesis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol 2012;32:65-73. 10.1161/ATVBAHA.111.239137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang LI, Mars WM, Michalopoulos GK. Signals and cells involved in regulating liver regeneration. Cells 2012;1:1261-92. 10.3390/cells1041261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su AI, Guidotti LG, Pezacki JP, et al. Gene expression during the priming phase of liver regeneration after partial hepatectomy in mice. Proc Natl Acad Sci U S A 2002;99:11181-6. 10.1073/pnas.122359899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White P, Brestelli JE, Kaestner KH, et al. Identification of transcriptional networks during liver regeneration. J Biol Chem 2005;280:3715-22. 10.1074/jbc.M410844200 [DOI] [PubMed] [Google Scholar]
- 29.Fausto N. Liver regeneration. J Hepatol 2000;32:19-31. 10.1016/S0168-8278(00)80412-2 [DOI] [PubMed] [Google Scholar]
- 30.Atri C, Guerfali FZ, Laouini D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci 2018;19:1801. 10.3390/ijms19061801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun R, Park O, Horiguchi N, et al. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology 2006;44:955-66. 10.1002/hep.21344 [DOI] [PubMed] [Google Scholar]
- 32.Gao B, Wang H, Lafdil F, et al. STAT proteins - key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J Hepatol 2012;57:430-41. 10.1016/j.jhep.2012.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streetz KL, Luedde T, Manns MP, et al. Interleukin 6 and liver regeneration. Gut 2000;47:309-12. 10.1136/gut.47.2.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazel Modares N, Polz R, Haghighi F, et al. IL-6 Trans-signaling Controls Liver Regeneration After Partial Hepatectomy. Hepatology 2019;70:2075-91. 10.1002/hep.30774 [DOI] [PubMed] [Google Scholar]
- 35.Li W, Liang X, Kellendonk C, et al. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem 2002;277:28411-7. 10.1074/jbc.M202807200 [DOI] [PubMed] [Google Scholar]
- 36.Blaschke F, Takata Y, Caglayan E, et al. Obesity, peroxisome proliferator-activated receptor, and atherosclerosis in type 2 diabetes. Arterioscler Thromb Vasc Biol 2006;26:28-40. 10.1161/01.ATV.0000191663.12164.77 [DOI] [PubMed] [Google Scholar]
- 37.Elcombe CR, Elcombe BM, Foster JR, et al. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats following dietary exposure to ammonium perfluorooctanoate occurs through increased activation of the xenosensor nuclear receptors PPARalpha and CAR/PXR. Arch Toxicol 2010;84:787-98. 10.1007/s00204-010-0572-2 [DOI] [PubMed] [Google Scholar]
- 38.Youssef J, Badr M. Enhanced hepatocarcinogenicity due to agonists of peroxisome proliferator-activated receptors in senescent rats: role of peroxisome proliferation, cell proliferation, and apoptosis. ScientificWorldJournal 2002;2:1491-500. 10.1100/tsw.2002.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters JM, Cheung C, Gonzalez FJ. Peroxisome proliferator-activated receptor-alpha and liver cancer: where do we stand? J Mol Med (Berl) 2005;83:774-85. 10.1007/s00109-005-0678-9 [DOI] [PubMed] [Google Scholar]
- 40.Shah YM, Morimura K, Yang Q, et al. Peroxisome proliferator-activated receptor alpha regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol Cell Biol 2007;27:4238-47. 10.1128/MCB.00317-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qu A, Jiang C, Cai Y, et al. Role of Myc in hepatocellular proliferation and hepatocarcinogenesis. J Hepatol 2014;60:331-8. 10.1016/j.jhep.2013.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nachmany I, Bogoch Y, Sivan A, et al. CD11b(+)Ly6G(+) myeloid-derived suppressor cells promote liver regeneration in a murine model of major hepatectomy. FASEB J 2019;33:5967-78. 10.1096/fj.201801733R [DOI] [PubMed] [Google Scholar]
- 43.Izumi T, Imai J, Yamamoto J, et al. Vagus-macrophage-hepatocyte link promotes post-injury liver regeneration and whole-body survival through hepatic FoxM1 activation. Nat Commun 2018;9:5300. 10.1038/s41467-018-07747-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishiyama K, Nakashima H, Ikarashi M, et al. Mouse CD11b+Kupffer Cells Recruited from Bone Marrow Accelerate Liver Regeneration after Partial Hepatectomy. PLoS One 2015;10:e0136774. 10.1371/journal.pone.0136774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massimi I, Pulcinelli FM, Piscitelli VP, et al. Non-steroidal anti-inflammatory drugs increase MRP4 expression in an endometriotic epithelial cell line in a PPARa dependent manner. Eur Rev Med Pharmacol Sci 2018;22:8487-96. [DOI] [PubMed] [Google Scholar]
- 46.Wen Y, Feng D, Wu H, et al. Defective Initiation of Liver Regeneration in Osteopontin-Deficient Mice after Partial Hepatectomy due to Insufficient Activation of IL-6/Stat3 Pathway. Int J Biol Sci 2015;11:1236-47. 10.7150/ijbs.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Czaja MJ, Geerts A, Xu J, et al. Monocyte chemoattractant protein 1 (MCP-1) expression occurs in toxic rat liver injury and human liver disease. J Leukoc Biol 1994;55:120-6. 10.1002/jlb.55.1.120 [DOI] [PubMed] [Google Scholar]
- 48.Marra F, DeFranco R, Grappone C, et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol 1998;152:423-30. [PMC free article] [PubMed] [Google Scholar]
- 49.Marino S, Cilfone NA, Mattila JT, et al. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect Immun 2015;83:324-38. 10.1128/IAI.02494-14 [DOI] [PMC free article] [PubMed] [Google Scholar]