Abstract
Objective
Posterior pelvic exenteration (PPE) can be required to achieve complete resection in ovarian cancer (OC) patients with large pelvic disease. This study aimed to analyze morbidity, complete resection rate, and survival of PPE.
Methods
Ninety patients who underwent PPE in our Comprehensive Cancer Center between January 2010 and February 2021 were retrospectively identified. To analyze practice evolution, 2 periods were determined: P1 from 2010 to 2017 and P2 from 2018 to 2021.
Results
A 82.2% complete resection rate after PPE was obtained, with rectal anastomosis in 96.7% of patients. Complication rate was at 30% (grade 3 in 9 patients), without significant difference according to periods or quality of resection. In a binary logistic regression adjusted on age and stoma, only age of 51–74 years old was associated with a lower rate of complication (odds ratio=0.223; p=0.026). Median overall and disease-free survivals (OS and DFS) from initial diagnosis were 75.21 and 29.84 months, respectively. A negative impact on OS and DFS was observed in case of incomplete resection, and on DFS in case of final cytoreductive surgery (FCS: after ≥6 chemotherapy cycles). Age ≥75-years had a negative impact on DFS for new OC surgery. For patients with complete resection, OS and DFS were decreased in case of interval cytoreductive surgery and FCS in comparison with primary cytoreductive surgery.
Conclusion
PPE is an effective surgical measure to achieve complete resection for a majority of patients. High rate of colorectal anastomosis was achieved without any mortality, with acceptable morbidity and high protective stoma rate.
Keywords: Pelvic Exenteration, Ovarian Cancer, Surgery, Survival, Prognosis
Synopsis
Posterior-pelvic-exenteration (PPE) can be required to achieve complete cyto-reductive-surgery (CS) in ovarian cancer (OC) patients. A 82.2% complete-CS rate was obtained for PPE, with rectal anastomosis in 96.7%. Complication rate was 30%. Negative impact on DFS for patients with incomplete-CS or final-CS or age ≥75-years for new OC and PPE.
INTRODUCTION
Ovarian cancer (OC) represents the most lethal tumor among gynecologic malignancies. It accounts for about 295,000 new cases and 185,000 deaths worldwide yearly [1]. This high mortality rate can be attributed to advanced stages at diagnosis in 60% of patients [2]. The standard treatment remains primary cytoreductive surgery (PCS) with the intent of complete resection followed by adjuvant chemotherapy. For patients without possible initial complete resection, interval cytoreductive surgery (ICS) after 3 cycles of neoadjuvant chemotherapy is proposed when a complete resection appears feasible [3,4,5]. When complete resection is considered as not feasible during PCS or ICS, surgery can be performed after 6 or more cycles of chemotherapy as final cytoreductive surgery (FCS) or late ICS [6,7]. Although the positive effect of platinum-based chemotherapy on the survival of patients with advanced ovarian carcinoma was widely accepted, the relative effect of aggressive surgical intervention on long-term outcomes has been more difficult to quantify. The clarification of the independent contribution of both surgery and platinum-based chemotherapy to the overall survival (OS) of patients with advanced ovarian carcinoma occurred in 2002 and was integrated into international practices since 2005 in most developed countries [8]. Patients who achieve a status of no residual disease after primary debulking surgery have a superior median survival (80 to 100 months), compared to patients with 0.1 to 1.0 cm of residual disease (50 months) or patients with more than 1.0 cm of residual disease (35 months) [9]. Consequently, the main objective of surgery for OC is to achieve complete resection of the disease. For patients with large pelvic disease, en-bloc resection of uterus and rectum can be required to achieve complete resection with posterior pelvic exenteration (PPE) [8,10,11,12,13,14]. PPE can be required for large pelvic recurrences [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Surgical morbidity of PPE has been previously reported [27,28,29,30], with documentation regarding protective stoma rates, using lateral colostomy or ileostomy. Mortality results were only partially reported in recent studies, with OS estimates ranging between 33 months and 49.4 months [31,32]. This study aimed to analyze a large experience of this surgical procedure in terms of morbidity, complete resection rate, and survival.
MATERIALS AND METHODS
1. Study design and data source
Consecutive patients with OC who underwent PPE in our Comprehensive Cancer Center (Marseille, France), between January 2010 and February 2021 were retrospectively identified. Patients received surgery at diagnosis (PCS) or after 3–4 chemotherapy cycles (ICS), or after 6 or more chemotherapy cycles (FCS), or for OC recurrence. After 3–4 chemotherapy cycles, re-evaluation was realized with CA-125 level decrease, computed tomography scan and laparoscopy to determine the possibility of complete surgical resection. For patients without possible complete resection, 2–3 new chemotherapy cycles were administered, and novel re-evaluation was performed to assess FCS feasibility. All patients underwent surgery and PPE with the intent to achieve complete resection. For primary OC, surgical procedures included hysterectomy with bilateral salpingo-oophorectomy, omentectomy, para-aortic, and pelvic lymph node dissection for patients with complete resection and from the year 2012 according to randomization in CARACO trial-NCT01218490 (lymph node dissection or not) [33] and appendectomy. Bowel resections other than rectal, diaphragmatic, and/or splenic resections were performed only if it was required to achieve complete resection.
To analyze practice evolution, 2 periods were determined: P1 from January 2010 to December 2017 and P2 from January 2018 to February 2021 (hyperthermic intraperitoneal chemotherapy [HIPEC] was performed only during P2). The following parameters were analyzed: age at surgery, American Society of Anesthesiologists (ASA) status, body mass index (BMI), dates of initial diagnosis and surgery, neoadjuvant chemotherapy cycles numbers, OC characteristics (histology, disease stage, primary OC or recurrence), surgical procedures (complete resection R0 or residual disease ≥R1, colorectal anastomosis and protective stoma with colostomy or ileostomy, date of stoma closure, duration of surgery, HIPEC), complications rates, complications grade (collected according to the Clavien-Dindo classification [34]), and post-operative hospitalization length (POHL). According to European guidelines [5], post-therapeutic follow-up included physical examination associated with CA125 serum level measurements every 3 months for 2 years, then every 6 months for the next 3 years, and annually thereafter. Computed tomography scan was usually performed 6 months after the end of chemotherapy, then each year in the absence of clinical symptoms or increase of CA125 serum level. Oncological outcomes were estimated with OS and disease-free survival (DFS). OS was defined as the time from initial diagnosis or surgery, to the date of death from any cause. DFS was defined as the time from initial diagnosis or surgery, to the date of recurrence or death from any cause. Patients without events were censored at the time of the last follow-up. This work was approved by our Institutional Review Board (PPE-OVC-IPC 2021-035).
2. Statistical analysis
Categorical variables were described using counts and frequencies, and quantitative variables were described using medians and ranges. Patients' characteristics according to periods and complete resection were compared using χ2 test or Fischer test. Quantitative values were compared using t-test. Significant factors associated with stoma and complications were determined using binary logistic regressions. Comparisons of survival results were analyzed with Log-rank test and multivariate Cox regression model. The level of statistical significance was set at α=0.05. Statistical analyses were carried out with the SPSS® software version 16 (Chicago, IL, USA).
RESULTS
During the study period, PPE was performed for 90 patients by 6 different surgeons: 36 during P1 and 54 during P2. Patient and surgery characteristics are reported according to periods in Table 1, and according to quality of resection (R0 or ≥R1) in Table S1. Median, mean, 95% confidence interval (CI) and range are reported for quantitative variables in Table 2.
Table 1. Characteristics of patients and surgery according to periods.
| Characteristics | P1 (n=36) | P2 (n=54) | χ2 | Total (n=90) | |
|---|---|---|---|---|---|
| No. (%) | No. (%) | p-value | No. (%) | ||
| Sequence | 0.021 | ||||
| Primary CS | 14 (38.9) | 19 (35.2) | 33 (36.7) | ||
| Interval CS | 10 (27.8) | 15 (27.8) | 25 (27.8) | ||
| Final CS | 11 (30.6) | 7 (13.0) | 18 (20.0) | ||
| Recurrence | 1 (2.8) | 13 (24.1) | 14 (15.6) | ||
| Histology | 0.221 | ||||
| High-grade serous | 30 (83.3) | 38 (70.4) | 68 (75.6) | ||
| Low-grade serous | 2 (5.6) | 2 (3.7) | 4 (4.4) | ||
| Others | 4 (11.1) | 14 (25.9) | 18 (20.0) | ||
| ASA status | 0.792 | ||||
| 1 | 4 (11.1) | 7 (13.0) | 11 (12.2) | ||
| 2 | 27 (75.0) | 37 (68.5) | 64 (71.1) | ||
| 3 | 5 (13.9) | 10 (18.5) | 15 (16.7) | ||
| R0/≥R1 | 0.310 | ||||
| R0 | 31 (86.1) | 43 (79.6) | 74 (82.2) | ||
| ≥R1 | 5 (13.9) | 11 (20.4) | 16 (17.8) | ||
| Rectal anastomosis | 0.211 | ||||
| No | 0 (0.0) | 3 (5.6) | 3 (3.3) | ||
| Yes | 36 (100.0) | 51 (94.4) | 87 (96.7) | ||
| Stoma | 0.020 | ||||
| No | 20 (55.6) | 17 (31.5) | 37 (41.1) | ||
| Yes | 16 (44.4) | 37 (68.5) | 53 (58.9) | ||
| Type of stoma | 0.045 | ||||
| Colostomy | 5 (31.2) | 3 (8.1) | 8 (15.1) | ||
| Ileostomy | 11 (68.8) | 34 (91.9) | 45 (94.9) | ||
| Surgeon | <0.001 | ||||
| 1 | 12 (33.3) | 10 (18.5) | 22 (24.4) | ||
| 2 | 9 (25.0) | 18 (33.3) | 27 (30.0) | ||
| 3 | 1 (2.8) | 14 (25.9) | 15 (16.7) | ||
| 4 | 1 (2.8) | 8 (14.8) | 9 (10.0) | ||
| 5 | 6 (16.7) | 4 (7.4) | 10 (11.1) | ||
| 6 | 7 (19.4) | 0 (0.0) | 7 (7.8) | ||
| Complication | 0.447 | ||||
| No | 26 (72.2) | 37 (68.5) | 63 (70.0) | ||
| Yes | 10 (27.8) | 17 (31.5) | 27 (30.0) | ||
| Grade complication | 0.180 | ||||
| 1 | 4 (40.0) | 6 (35.3) | 10 (37.0) | ||
| 2 | 1 (10.0) | 7 (41.2) | 8 (29.6) | ||
| 3 | 5 (50.0) | 4 (23.5) | 9 (33.3) | ||
| Reoperation | 0.257 | ||||
| No | 31 (86.1) | 50 (92.6) | 81 (90.0) | ||
| Yes | 5 (13.9) | 4 (7.4) | 9 (10.0) | ||
| Stoma closure | 0.522 | ||||
| No | 5 (31.2) | 13 (35.1) | 18 (34.0) | ||
| Yes | 11 (68.8) | 24 (64.9) | 35 (66.0) | ||
| HIPEC | 0.041 | ||||
| No | 36 (100.0) | 48 (88.9) | 84 (93.3) | ||
| Yes | 0 (0.0) | 6 (11.1) | 6 (6.7) | ||
| BMI | 0.085 | ||||
| <30 | 29 (80.6) | 50 (92.6) | 79 (87.8) | ||
| ≥30 | 7 (19.4) | 4 (7.4) | 11 (12.2) | ||
| Age (yr) | 0.747 | ||||
| ≤50 | 6 (16.7) | 6 (11.1) | 12 (13.3) | ||
| 51–74 | 24 (66.7) | 38 (70.4) | 62 (68.9) | ||
| ≥75 | 6 (16.7) | 10 (18.5) | 16 (17.8) | ||
| Duration surgery (min) | 0.382 | ||||
| ≤350 | 20 (55.6) | 27 (50.0) | 47 (52.2) | ||
| >350 | 16 (44.4) | 27 (50.0) | 43 (47.8) | ||
ASA, American Society of Anesthesiologists; BMI, body mass index; CS, caesarean section; HIPEC, hyperthermic intraperitoneal chemotherapy.
Table 2. Median, mean, 95% CI, and range for quantitative variables.
| Characteristics | Median | Mean | 95% CI | Range | p-value (t-test) | |
|---|---|---|---|---|---|---|
| Age (yr) | 66 | 63.7 | 61.6–65.9 | 32–79 | ||
| BMI (kg/m2) | 23.2 | 24.0 | 23.1–25.0 | 16.9–42.6 | ||
| POLH (days) | 11 | 12.3 | 11.4–13.3 | 5–35 | ||
| Duration of surgery (min) | 350 | 351 | 331–371 | 167–613 | ||
| Time to stoma closure (mo) | 3.93 | 4.23 | 3.46–4.99 | 0.27–10.73 | ||
| Age (yr) | 0.094 | |||||
| P1 | 62.5 | 61.8 | 57.9–65.6 | 32–79 | ||
| P2 | 66.0 | 65.0 | 62.5–67.6 | 39–78 | ||
| BMI (kg/m2) | 0.147 | |||||
| P1 | 22.90 | 24.38 | 22.65–26.10 | 17.2–37.4 | ||
| P2 | 23.27 | 23.83 | 22.62–25.03 | 16.9–42.6 | ||
| POLH (days) | 0.606 | |||||
| P1 | 11 | 12.0 | 10.5–13.5 | 5–23 | ||
| P2 | 12 | 12.5 | 11.3–13.8 | 6–35 | ||
| Duration of surgery (min) | 0.002 | |||||
| P1 | 343 | 340 | 320–360 | 180–460 | ||
| P2 | 352 | 357 | 326–389 | 167–613 | ||
| Time to stoma closure (mo) | 0.016 | |||||
| P1 | 3.70 | 4.76 | 2.68–6.85 | 0.27–10.73 | ||
| P2 | 3.98 | 3.98 | 3.26–4.69 | 0.43–8.20 | ||
| POLH (days) | <0.001 | |||||
| No complication | 11 | 11.1 | 10.4–11.9 | 5–19 | ||
| Complication | 14 | 15.1 | 12.8–17.4 | 7–35 | ||
BMI, body mass index; CI, confidence interval; POLH, post-operative length of hospitalization.
Periods of treatment: significant differences were observed between P1 and P2 in treatment sequences, use of HIPEC, and the number of procedures for each surgeon (Table 1). Increased duration of surgery was also reported during P2 (6 HIPEC procedures during P2, 0 during P1) (Table 2). No statistically significant difference was observed for all factors between R0 and ≥R1 resections (Table S1).
Protective stoma rate was higher during P2 (68.5%) in comparison with P1 (44.4%) (p=0.020) with more ileostomy during P2 (91.9% versus 68.8%) (Table 1). Significantly higher rates in stoma use were observed for age, periods, and surgeons in univariate analysis (results were not significant for ASA status, BMI, HIPEC, R0 or ≥R1 resection, histology, and time of surgery). In binary logistic regression adjusted on age and periods, only P2 was associated with a higher rate of stoma (odds ratio [OR]=2.979; p=0.019) (Table 3). Stoma closure rate was up to 66% at a median time of 3.93 months without difference between periods and quality of resection complete or not.
Table 3. Factors associated with stoma and with complications in binary logistic regression analysis.
| Factors | OR | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Factors associated with stoma | |||||
| Age (yr) | |||||
| ≤50 | 1 | ||||
| 51–74 | 0.448 | 0.115–1.739 | 0.246 | ||
| ≥75 | 1.962 | 0.329–11.70 | 0.459 | ||
| Periods | |||||
| P1 | 1 | ||||
| P2 | 2.979 | 1.19–7.44 | 0.019 | ||
| Factors associated with complications | |||||
| Age (yr) | |||||
| ≤50 | 1 | ||||
| 51–74 | 0.223 | 0.060–0.834 | 0.026 | ||
| ≥75 | 0.368 | 0.077–1.770 | 0.212 | ||
| Stoma | |||||
| No | 1 | ||||
| Yes | 2.353 | 0.829–6.676 | 0.108 | ||
CI, confidence interval; OR, odds ratio.
Duration of surgery in the global cohort was 350 minutes (median, 95% CI=331–371). Increased duration was observed during P2 versus P1 (Table 3). Six patients received HIPEC during P2 but none during P1.
The complication rate was up to 30%, without statistically significant difference according to periods or quality of resection. Statistically significant differences were observed according to protective stoma use (37.7% [20/53] with versus 18.9% [7/37] without protective stoma, p=0.045) and according to age (58.3%, 22.6% and 37.5% for patients ≤50, 51–74 and ≥75 years old, respectively, p=0.036) (Table 4). Grade 3 complications were observed for 9 patients with re-operation (10%): 13.9% during P1, and 7.4% during P2. Seven anastomotic fistula and one pelvic infected collection were observed: 2 anastomotic fistula (1 grade 3 and 1 grade 1) and 1 pelvic sepsis (grade 3) for patients without stoma (3/37), 5 anastomotic fistula (5 grade 3) for patients with protective stoma (5/53). In a binary logistic regression adjusted on age and stoma, only age of 51–74 years old was associated with a lower rate of complication (OR=0.223; p=0.026) (Table 3). Median POHL was 11 days, without difference between periods. Higher POHL was observed in patients with complications (p<0.001) (Table 3).
Table 4. Complications and grade of complications.
| Complications | No | Yes | χ2/Fisher | |
|---|---|---|---|---|
| No. (%) | No. (%) | p-value | ||
| Periods | 0.447 | |||
| P1 | 26 (41.3) | 10 (37.0) | ||
| P2 | 37 (58.7) | 17 (63.0) | ||
| Sequence | 0.642 | |||
| Primary CS | 22 (34.9) | 11 (40.7) | ||
| Interval CS | 20 (31.7) | 5 (18.5) | ||
| Final CS | 12 (19.0) | 6 (22.2) | ||
| Recurrence | 9 (14.3) | 5 (18.5) | ||
| Histology | 0.926 | |||
| High grade serous | 48 (76.2) | 20 (74.1) | ||
| Low grade serous | 3 (4.8) | 1 (3.7) | ||
| Others | 12 (19.0) | 6 (22.2) | ||
| ASA status | 0.256 | |||
| 1 | 6 (9.5) | 5 (18.5) | ||
| 2 | 48 (76.2) | 16 (59.3) | ||
| 3 | 9 (14.3) | 6 (22.2) | ||
| R0/≥R1 | 0.439 | |||
| R0 | 51 (81.0) | 23 (85.2) | ||
| ≥R1 | 12 (19.0) | 4 (14.8) | ||
| Rectal anastomosis | 0.662 | |||
| No | 2 (3.2) | 1 (3.7) | ||
| Yes | 61 (96.8) | 26 (96.3) | ||
| Stoma | 0.045 | |||
| No | 30 (47.6) | 7 (25.9) | ||
| Yes | 33 (52.4) | 20 (74.1) | ||
| Type stoma | 0.345 | |||
| Colostomy | 4 (12.1) | 4 (20.0) | ||
| Ileostomy | 29 (87.9) | 16 (80.0) | ||
| Surgeon | 0.303 | |||
| 1 | 13 (20.6) | 9 (33.3) | ||
| 2 | 20 (31.7) | 7 (25.9) | ||
| 3 | 9 (14.3) | 6 (22.2) | ||
| 4 | 9 (14.3) | 0 (0.0) | ||
| 5 | 7 (11.1) | 3 (11.1) | ||
| 6 | 5 (7.9) | 2 (7.4) | ||
| Stoma closure | 0.334 | |||
| No | 10 (30.3) | 8 (40.0) | ||
| Yes | 23 (69.7) | 12 (60.0) | ||
| HIPEC | 0.414 | |||
| No | 58 (92.1) | 26 (96.3) | ||
| Yes | 5 (7.9) | 1 (3.7) | ||
| BMI | 0.297 | |||
| <30 | 54 (85.7) | 25 (92.6) | ||
| ≥30 | 9 (14.3) | 2 (7.4) | ||
| Age (yr) | 0.036 | |||
| ≤50 | 5 (7.9) | 7 (25.9) | ||
| 51–74 | 48 (76.2) | 14 (51.9) | ||
| ≥75 | 10 (15.9) | 6 (22.2) | ||
| Duration surgery (min) | 0.572 | |||
| ≤350 | 33 (52.4) | 14 (51.9) | ||
| >350 | 30 (47.6) | 13 (48.1) | ||
ASA, American Society of Anesthesiologists; BMI, body mass index; CS, caesarean section; HIPEC, hyperthermic intraperitoneal chemotherapy.
Oncological outcome: Median follow-ups from initial diagnosis and surgery were 75.31 and 53.77 months, respectively. Median OS and DFS for all patients and for patients with primary OC surgery (excluding recurrences) are reported in Table S2. OS and DFS at 3-years and 5-years are reported in Table S2. In univariate analysis, statistically significant differences were observed according to quality of resection, age groups, and sequence of treatment for OS and DFS from initial diagnosis. In multivariate Cox analysis, for all patients, OS from initial diagnosis was significantly decreased only for patients without complete resection (hazard ratio [HR]=4.309; p=0.004). DFS from initial diagnosis was significantly decreased for patients without complete resection (HR=3.742; p=0.001) and with FCS (HR=3.190; p=0.006) (Table 5). For patients with primary OC surgery, from initial diagnosis: OS was significantly decreased for patients without complete resection (HR=3.936; p=0.011) (Fig. S1A) and age (p=0.046) (Fig. S1B), and DFS was significantly decreased for patients without complete resection (HR=4.042; p=0.001), ≥75-years old (HR=4.488; p=0.040), and FCS (HR=2.325; p=0.043) (Table 5).
Table 5. Multivariate Cox analysis.
| Cox analysis results | HR | 95% CI | p-value | |||
|---|---|---|---|---|---|---|
| All patients | ||||||
| OS from initial diagnosis | ||||||
| Time of surgery | ||||||
| Primary CS | 1 | 0.064 | ||||
| Interval CS | 2.172 | 0.659–7.159 | 0.203 | |||
| Final CS | 2.212 | 0.747–6.550 | 0.152 | |||
| Recurrence | 0.501 | 0.131–1.919 | 0.313 | |||
| R0/≥R1 | ||||||
| R0 | 1 | |||||
| ≥R1 | 4.309 | 1.588–11.69 | 0.004 | |||
| Age (yr) | ||||||
| ≤50 | 1 | 0.027 | ||||
| 51–74 | 0.640 | 0.169–2.425 | 0.511 | |||
| ≥75 | 2.402 | 0.518–11.13 | 0.263 | |||
| DFS from initial diagnosis | ||||||
| Time of surgery | ||||||
| Primary CS | 1 | 0.002 | ||||
| Interval CS | 1.440 | 0.582–3.561 | 0.431 | |||
| Final CS | 3.190 | 1.400–7.268 | 0.006 | |||
| Recurrence | 0.666 | 0.290–1.527 | 0.337 | |||
| R0/≥R1 | ||||||
| R0 | 1 | |||||
| ≥R1 | 3.742 | 1.718–8.152 | 0.001 | |||
| Age (yr) | ||||||
| ≤50 | 1 | 0.141 | ||||
| 51–74 | 1.810 | 0.614–5.337 | 0.282 | |||
| ≥75 | 3.468 | 0.935–12.86 | 0.063 | |||
| Patients with primary ovarian cancer | ||||||
| OS from initial diagnosis | ||||||
| Time of surgery | ||||||
| Primary CS | 1 | 0.427 | ||||
| Interval CS | 1.775 | 0.549–5.747 | 0.338 | |||
| Final CS | 2.031 | 0.690–5.980 | 0.198 | |||
| R0/≥R1 | ||||||
| R0 | 1 | |||||
| ≥R1 | 3.936 | 1.378–11.24 | 0.011 | |||
| Age (yr) | ||||||
| ≤50 | 1 | 0.046 | ||||
| 51–74 | 1.083 | 0.227–5.182 | 0.920 | |||
| ≥75 | 3.635 | 0.642–20.59 | 0.145 | |||
| DFS from initial diagnosis | ||||||
| Time of surgery | ||||||
| Primary CS | 1 | 0.061 | ||||
| Interval CS | 0.980 | 0.404–2.379 | 0.964 | |||
| Final CS | 2.325 | 1.026–5.269 | 0.043 | |||
| R0/≥R1 | ||||||
| R0 | 1 | |||||
| ≥R1 | 4.042 | 1.772–9.219 | 0.001 | |||
| Age (yr) | ||||||
| ≤50 | 1 | 0.059 | ||||
| 51–74 | 1.861 | 0.540–6.412 | 0.325 | |||
| ≥75 | 4.488 | 1.075–18.74 | 0.040 | |||
CI, confidence interval; CS, caesarean section; DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
Multivariate Cox analysis among patients with complete resection (Table S3): OS from initial diagnosis was not different for age groups, but we observed a trend toward ICS (HR=4.048; 95% CI=0.958–17.10; p=0.057) without difference for FCS and recurrence compared to PCS. DFS from initial diagnosis was no different for age groups but we observed a significantly significant difference for ICS (HR=3.493; 95% CI=1.22–10.00; p=0.020) and FCS (HR=3.509; 95% CI=1.301–9.464; p=0.013) without difference for recurrence compared to PCS (Fig. S2).
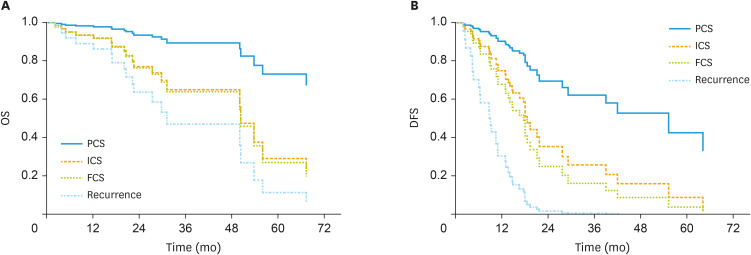
OS from surgery was not different for age groups but we observed a trend toward ICS (HR=3.926; 95% CI=0.937–16.45; p=0.061) and a significant difference for FCS (HR=4.116; 95% CI=1.075–15.76; p=0.039) and recurrence (HR=6.908; 95% CI=1.145–41.67; p=0.035) compared to PCS. DFS from surgery was no different for age groups but we observed a significant difference for ICS (HR=2.860; 95% CI=1.014–8.066; p=0.047), for FCS (HR=3.819; 95% CI=1.397–10.44; p=0.009) and recurrence (HR=11.629; 95% CI=3.969–34.07; p<0.001) compared to PCS (Fig. 1).
Fig. 1. (A) OS from surgery for patients with complete resection according to time of surgery adjusted on age groups. (B) DFS from surgery for patients with complete resection according to time of surgery adjusted on age groups.
DFS, disease-free survival; FCS, final cytoreductive surgery; ICS, interval cytoreductive surgery; OS, overall survival; PCS, primary cytoreductive surgery.
DISCUSSION
In summary, we report an 82.2% complete resection rate after PPE, without difference between the 2 considered periods, with rectal anastomosis in 96.7% of patients and protective stoma in 60.9% of these patients (53/87) with a significant increase of protective stoma rate during the second period (OR=2.979) and a significant increase of ileostomy. The overall complication rate was at 30% including 33.3% of grade 3 complications, with fewer complications in patients between 51 and 74-years old (OR=0.223). A negative impact on OS and DFS from initial diagnosis was observed for incomplete resection, and for FCS on DFS. Moreover, age ≥75-years old had a negative impact on DFS from diagnosis for new OC surgery. For patients with complete resection, OS and DFS were decreased for ICS and FCS in comparison with PCS.
We reported more PPE during the second period of 38 months in comparison with the first period of 96 months. This difference was not related to an increase in PPE indications but can be attributed to an increase in the number of patients treated for new stage III–IV OC, and to an increase of PPE for recurrent OC.
Time of surgery: There was no significant difference between the 2 periods, of PCS, ICS, and FCS, with more PPE for recurrent OCs during the second period. In our recently published large cohort of 1,260 patients who underwent surgery from 1993 to 2015 for new OC [35], we reported lower rate of ICS (14.1%: 178/1,260) than the 32.9% (25/76) reported in this study, 33.6% (423/1,260) of FCS versus 23.7% (18/76) here, and 52.3% (659/1,260) of PCS versus 43.4% (33/76) here. In our previous study examining PPE for OC between 1990 and 2004 [12], we reported 168 PCS (55.1%), 69 ICS (22.6%: 25.5% and 29.9% from 1995 to 1999 and from 2000 to 2004, respectively), 36 FCS (11.8%) and 32 recurrent OC (10.5%) PPE. These rates were different in the more recent study by Berretta et al. [13] that considered PPE performed between 2010 and 2014: 77% (17/22) PCS and 23% (5/22) ICS. Higher rates of ICS and FCS for surgery with PPE were reported in this study in comparison with others studies [12,13] for PPE and for surgery for new OC [35] to achieved complete resection in a high proportion of patients.
Complete resection rates were 64.7% (815/1,260) for patients who underwent surgery from 1993 to 2015 for new OC [35] in comparison with 58% (173/303) between 1990 and 2004 [12] and 82.2% (74/90) in the present study. In others works from authors focusing on PPE, complete resection rates were between 72.2% and 17.8% [12,17,18,19,20,24,27,36]. The higher rate of complete resection reported in the present study could be attributed to increased efficacy in pre-operative chemotherapy for ICS and FCS, as well as better evaluation of complete resection feasibility by laparoscopy after neo-adjuvant treatments, as well as a better selection of patients for the timing of surgery.
Colorectal anastomosis was performed in 99% (302/305) of patients from our previous PPE study [12], in all of the 22 patients included in Beretta et al. [13] and in 96.7% in the present study. After rectal anastomosis, 19.5% (59/302), 18.2% (4/22), and 60.9% (53/87) underwent protective stoma in studies reported by Houvenaeghel et al. [12], Berretta et al. [13], and by this study, respectively. In older other studies, protective stoma rates after PPE ranges were encompassed between 3.2% and 58.4% [10,12,17,18,20,24,27,36,37]. A higher rate of protective stoma is reported in our present study with a significant increase during the second period (OR=2.979). This higher rate of protective stoma was in relation with the intent to avoid severe complications due to anastomotic leakage. The complication rate for patients without protective stoma (18.9%: 7/37) was lower than the one observed for patients with protective stoma (20/53: 37.7%). This result might reflect the surgeon's choice to avoid protective stoma for patients with less aggressive surgery. We reported a higher rate of ileostomy versus colostomy (94.9%) compared with 64.4% (38/59) and 25.0% (1/4) in previous studies [12,13]. Moreover, the ileostomy rate increased significantly during the second period of the present study (91.9% versus 68.8%). This result is in relation with more simple surgery for stoma closure, as reported by Mourton et al. (17.1% of protective stoma: all with ileostomy) [27]. Stoma closure rate was at 66% in the present study and between 33.3% and 100% in previous studies [12,17,19,20,21,24,27].
No patients died during the 90-days of post-operative period. Similarly low or absence of early mortality (between 0% and 10%) was also reported by previous PPE studies including 20 to 265 patients [10,12,13,18,20,21,24,27,30,36,37].
Overall complication rates were up to 82% (18/22) [13] and 27.2% [12] in previous PPE studies and 30% in the present study. More comparable results can be report regarding major morbidity rate and/or grade ≥3 complications: 8.6% to 46% [10,12,17,18,19,20,21,27,36], 41% (9/22) [13] and 10% in the present study. Anastomotic fistulae rates were encompassed between 0% and 9.5% in previous studies [10,12,17,18,19,20,21,27,36,37]. The lower rate of grade 3 complications in the present study could be attributed to recent years' increasing efforts in patients management with pre-habilitation and enhanced recovery after surgery programs [38], but also higher rates of protective stoma. Consistently, POHL was decreased in the present study (median of 11 days), compared to older studies (median: 10.5 to 16 days). However, the only factor associated with decreased complication rate in this study was age between 51 and 74 years old (OR=0.223). Complications, particularly grade 3, can affect the delay between surgery and adjuvant chemotherapy, which is associated with shorter OS and DFS [39].
Oncologic outcome: Median OS after PPE of 53.77 months from surgery, and 75.31 months from initial diagnosis in the present study, were similar to previous studies (14 to 55 months according to the duration of follow-up, period of study, and number of patients analyzed) [10,12,13,18,20,21,27,31,32,36,40]. Median OS after surgery with complete resection for new OC (with exclusion of recurrent OC) was 55.1 months among the 1,260 patients who underwent surgery for stages III–IV epithelial ovarian cancer [35] and 75.31 months in the present study, with better results for patients younger than 74-years old. Median DFS after PPE in previous studies, 14 to 50 months [13,31,32,40], was close to the one we are reporting here: 16.62 months from surgery, and 29.84 months from initial diagnosis. After complete resection, which remains the most powerful prognosis factor, we observed shorter DFS from surgery for ICS, FCS, and recurrence in comparison with PCS, as similarly reported by Delga et al. [35]. However, the CHORUS trial [4] showed similar median OS and DFS for PCS and ICS (22.6 versus 24 months, and 12 versus 10.7, respectively) but the rate of complete resection was lower (17% and 39% for PCS and ICS, respectively). Moreover, comparable survival rates between FCS and others time of surgery were reported in several studies [3,7,8]. The less favorable OS and DFS results in the present study for ICS, and FCS after PPE with complete resection might be related to more severe diseases at diagnosis, as well as less favorable chemotherapy responses.
The mains limitations of the current study were the retrospective design, and missing details regarding chemotherapy regimen and additional adjuvant treatment after adjuvant or neoadjuvant chemotherapy and surgery. However, the combination of platinum agent and taxanes regimens were integrated in international practices and used since the year 2000.
In conclusion, the survival results for PPE presented in this study, in comparison with previous studies results with surgery for OC with or without PPE for majority of patients, supports PPE as an effective surgery with intent to achieve complete resection for the great majority of patients. Median OS was increased in this recent study compared to older ones, probably due to several points: high rate of complete resection, more efficient chemotherapy regimen in comparison with older studies, improvement in patient's selection and time of surgery. A high rate of colorectal anastomosis was achieved without any mortality, with acceptable morbidity and a high protective stoma rate.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: H.G.
- Data curation: H.G.
- Formal analysis: H.G.
- Investigation: H.G.
- Methodology: H.G.
- Validation: H.G., D.N.A., B.G., B.M., J.C., M.D., S.L.
- Visualization: H.G.
- Writing - original draft: H.G., D.N.A., B.G., B.M., J.C., M.D., S.L.
- Writing - review & editing: H.G., D.N.A., B.G., B.M., J.C., M.D., S.L.
SUPPLEMENTARY MATERIALS
Characteristics of patients and surgery according to quality of resection (R0 or ≥R1)
Survival outcome in univariate analysis
Multivariate Cox analysis among patients with complete resection
(A) OS from initial diagnosis for patients with primary ovarian cancer according to quality of resection (R0 or ≥R1) adjusted on age, time of surgery and quality of resection. (B) OS from initial diagnosis for patients with primary ovarian cancer according to age, adjusted on age, time of surgery and quality of resection.
(A) OS from initial diagnosis for patients with complete resection according to time of surgery adjusted on age groups. (B) DFS from initial diagnosis for patients with complete resection according to time of surgery adjusted on age groups.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 4.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 5.Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 6.Stoeckle E, Bourdarias L, Guyon F, Croce S, Brouste V, Thomas L, et al. Progress in survival outcomes in patients with advanced ovarian cancer treated by neo-adjuvant platinum/taxane-based chemotherapy and late interval debulking surgery. Ann Surg Oncol. 2014;21:629–636. doi: 10.1245/s10434-013-3278-x. [DOI] [PubMed] [Google Scholar]
- 7.da Costa Miranda V, de Souza Fêde ÂB, Dos Anjos CH, da Silva JR, Sanchez FB, da Silva Bessa LR, et al. Neoadjuvant chemotherapy with six cycles of carboplatin and paclitaxel in advanced ovarian cancer patients unsuitable for primary surgery: Safety and effectiveness. Gynecol Oncol. 2014;132:287–291. doi: 10.1016/j.ygyno.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 9.Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT) Gynecol Oncol. 2012;124:10–14. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Bristow RE, del Carmen MG, Kaufman HS, Montz FJ. Radical oophorectomy with primary stapled colorectal anastomosis for resection of locally advanced epithelial ovarian cancer. J Am Coll Surg. 2003;197:565–574. doi: 10.1016/S1072-7515(03)00478-2. [DOI] [PubMed] [Google Scholar]
- 11.Hudson CN. A radical operation for fixed ovarian tumours. J Obstet Gynaecol Br Commonw. 1968;75:1155–1160. doi: 10.1111/j.1471-0528.1968.tb02901.x. [DOI] [PubMed] [Google Scholar]
- 12.Houvenaeghel G, Gutowski M, Buttarelli M, Cuisenier J, Narducci F, Dalle C, et al. Modified posterior pelvic exenteration for ovarian cancer. Int J Gynecol Cancer. 2009;19:968–973. doi: 10.1111/IGC.0b013e3181a7f38b. [DOI] [PubMed] [Google Scholar]
- 13.Berretta R, Marchesi F, Volpi L, Ricotta G, Monica M, Sozzi G, et al. Posterior pelvic exenteration and retrograde total hysterectomy in patients with locally advanced ovarian cancer: clinical and functional outcome. Taiwan J Obstet Gynecol. 2016;55:346–350. doi: 10.1016/j.tjog.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Eisenkop SM, Nalick RH, Teng NN. Modified posterior exenteration for ovarian cancer. Obstet Gynecol. 1991;78:879–885. [PubMed] [Google Scholar]
- 15.Morris M, Gershenson DM, Wharton JT, Copeland LJ, Edwards CL, Stringer CA. Secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Gynecol Oncol. 1989;34:334–338. doi: 10.1016/0090-8258(89)90168-6. [DOI] [PubMed] [Google Scholar]
- 16.Moutardier V, Houvenaeghel G, Lelong B, Mokart D, Delpero JR. Colorectal function preservation in posterior and total supralevator exenteration for gynecologic malignancies: an 89-patient series. Gynecol Oncol. 2003;89:155–159. doi: 10.1016/s0090-8258(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 17.Spirtos NM, Eisenkop SM, Schlaerth JB, Ballon SC. Second-look laparotomy after modified posterior exenteration: patterns of persistence and recurrence in patients with stage III and stage IV ovarian cancer. Am J Obstet Gynecol. 2000;182:1321–1327. doi: 10.1067/mob.2000.106250. [DOI] [PubMed] [Google Scholar]
- 18.Clayton RD, Obermair A, Hammond IG, Leung YC, McCartney AJ. The Western Australian experience of the use of en bloc resection of ovarian cancer with concomitant rectosigmoid colectomy. Gynecol Oncol. 2002;84:53–57. doi: 10.1006/gyno.2001.6469. [DOI] [PubMed] [Google Scholar]
- 19.Scarabelli C, Gallo A, Franceschi S, Campagnutta E, De G, Giorda G, et al. Primary cytoreductive surgery with rectosigmoid colon resection for patients with advanced epithelial ovarian carcinoma. Cancer. 2000;88:389–397. doi: 10.1002/(sici)1097-0142(20000115)88:2<389::aid-cncr21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Obermair A, Hagenauer S, Tamandl D, Clayton RD, Nicklin JL, Perrin LC, et al. Safety and efficacy of low anterior en bloc resection as part of cytoreductive surgery for patients with ovarian cancer. Gynecol Oncol. 2001;83:115–120. doi: 10.1006/gyno.2001.6353. [DOI] [PubMed] [Google Scholar]
- 21.Bridges JE, Leung Y, Hammond IG, McCartney AJ. En bloc resection of epithelial ovarian tumors with concomitant rectosigmoid colectomy: the KEMH experience. Int J Gynecol Cancer. 1993;3:199–202. doi: 10.1046/j.1525-1438.1993.03040199.x. [DOI] [PubMed] [Google Scholar]
- 22.Barnes W, Johnson J, Waggoner S, Barter J, Potkul R, Delgado G. Reverse hysterocolposigmoidectomy (RHCS) for resection of panpelvic tumors. Gynecol Oncol. 1991;42:151–155. doi: 10.1016/0090-8258(91)90336-4. [DOI] [PubMed] [Google Scholar]
- 23.Sainz de la Cuesta R, Goodman A, Halverson SS. En bloc pelvic peritoneal resection of the intraperitoneal pelvic viscera in patients with advanced epithelial ovarian cancer. Cancer J Sci Am. 1996;2:152–157. [PubMed] [Google Scholar]
- 24.Hertel H, Diebolder H, Herrmann J, Köhler C, Kühne-Heid R, Possover M, et al. Is the decision for colorectal resection justified by histopathologic findings: a prospective study of 100 patients with advanced ovarian cancer. Gynecol Oncol. 2001;83:481–484. doi: 10.1006/gyno.2001.6338. [DOI] [PubMed] [Google Scholar]
- 25.Eisenkop SM, Friedman RL, Spirtos NM. The role of secondary cytoreductive surgery in the treatment of patients with recurrent epithelial ovarian carcinoma. Cancer. 2000;88:144–153. doi: 10.1002/(sici)1097-0142(20000101)88:1<144::aid-cncr20>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Gronlund B, Lundvall L, Christensen IJ, Knudsen JB, Høgdall C. Surgical cytoreduction in recurrent ovarian carcinoma in patients with complete response to paclitaxel-platinum. Eur J Surg Oncol. 2005;31:67–73. doi: 10.1016/j.ejso.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Mourton SM, Temple LK, Abu-Rustum NR, Gemignani ML, Sonoda Y, Bochner BH, et al. Morbidity of rectosigmoid resection and primary anastomosis in patients undergoing primary cytoreductive surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2005;99:608–614. doi: 10.1016/j.ygyno.2005.07.112. [DOI] [PubMed] [Google Scholar]
- 28.Berek JS, Hacker NF, Lagasse LD. Rectosigmoid colectomy and reanastomosis to facilitate resection of primary and recurrent gynecologic cancer. Obstet Gynecol. 1984;64:715–720. [PubMed] [Google Scholar]
- 29.Mirhashemi R, Averette HE, Estape R, Angioli R, Mahran R, Mendez L, et al. Low colorectal anastomosis after radical pelvic surgery: a risk factor analysis. Am J Obstet Gynecol. 2000;183:1375–1379. doi: 10.1067/mob.2000.110908. [DOI] [PubMed] [Google Scholar]
- 30.Sonnendecker EW, Beale PG. Rectosigmoid resection without colostomy during primary cytoreductive surgery for ovarian carcinoma. Int Surg. 1989;74:10–12. [PubMed] [Google Scholar]
- 31.Tixier H, Fraisse J, Chauffert B, Mayer F, Causeret S, Loustalot C, et al. Evaluation of pelvic posterior exenteration in the management of advanced-stage ovarian cancer. Arch Gynecol Obstet. 2010;281:505–510. doi: 10.1007/s00404-009-1175-0. [DOI] [PubMed] [Google Scholar]
- 32.Revaux A, Rouzier R, Ballester M, Selle F, Daraï E, Chéreau E. Comparison of morbidity and survival between primary and interval cytoreductive surgery in patients after modified posterior pelvic exenteration for advanced ovarian cancer. Int J Gynecol Cancer. 2012;22:1349–1354. doi: 10.1097/IGC.0b013e318265d358. [DOI] [PubMed] [Google Scholar]
- 33.Classe JM, Cerato E, Boursier C, Dauplat J, Pomel C, Villet R, et al. Retroperitoneal lymphadenectomy and survival of patients treated for an advanced ovarian cancer: the CARACO trial. J Gynecol Obstet Biol Reprod (Paris) 2011;40:201–204. doi: 10.1016/j.jgyn.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 35.Delga B, Classe JM, Houvenaeghel G, Blache G, Sabiani L, El Hajj H, et al. 30 Years of experience in the management of stage III and IV epithelial ovarian cancer: impact of surgical strategies on survival. Cancers (Basel) 2020;12:E768. doi: 10.3390/cancers12030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soper JT, Couchman G, Berchuck A, Clarke-Pearson D. The role of partial sigmoid colectomy for debulking epithelial ovarian carcinoma. Gynecol Oncol. 1991;41:239–244. doi: 10.1016/0090-8258(91)90316-w. [DOI] [PubMed] [Google Scholar]
- 37.Guidozzi F, Ball JH. Extensive primary cytoreductive surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 1994;53:326–330. doi: 10.1006/gyno.1994.1142. [DOI] [PubMed] [Google Scholar]
- 38.de Nonneville A, Jauffret C, Braticevic C, Cecile M, Faucher M, Pouliquen C, et al. Enhanced recovery after surgery program in older patients undergoing gynaecologic oncological surgery is feasible and safe. Gynecol Oncol. 2018;151:471–476. doi: 10.1016/j.ygyno.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Mahner S, Eulenburg C, Staehle A, Wegscheider K, Reuss A, Pujade-Lauraine E, et al. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: analysis of prospective randomised phase III trials. Eur J Cancer. 2013;49:142–149. doi: 10.1016/j.ejca.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 40.Rose PG, Nerenstone S, Brady MF, Clarke-Pearson D, Olt G, Rubin SC, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med. 2004;351:2489–2497. doi: 10.1056/NEJMoa041125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of patients and surgery according to quality of resection (R0 or ≥R1)
Survival outcome in univariate analysis
Multivariate Cox analysis among patients with complete resection
(A) OS from initial diagnosis for patients with primary ovarian cancer according to quality of resection (R0 or ≥R1) adjusted on age, time of surgery and quality of resection. (B) OS from initial diagnosis for patients with primary ovarian cancer according to age, adjusted on age, time of surgery and quality of resection.
(A) OS from initial diagnosis for patients with complete resection according to time of surgery adjusted on age groups. (B) DFS from initial diagnosis for patients with complete resection according to time of surgery adjusted on age groups.