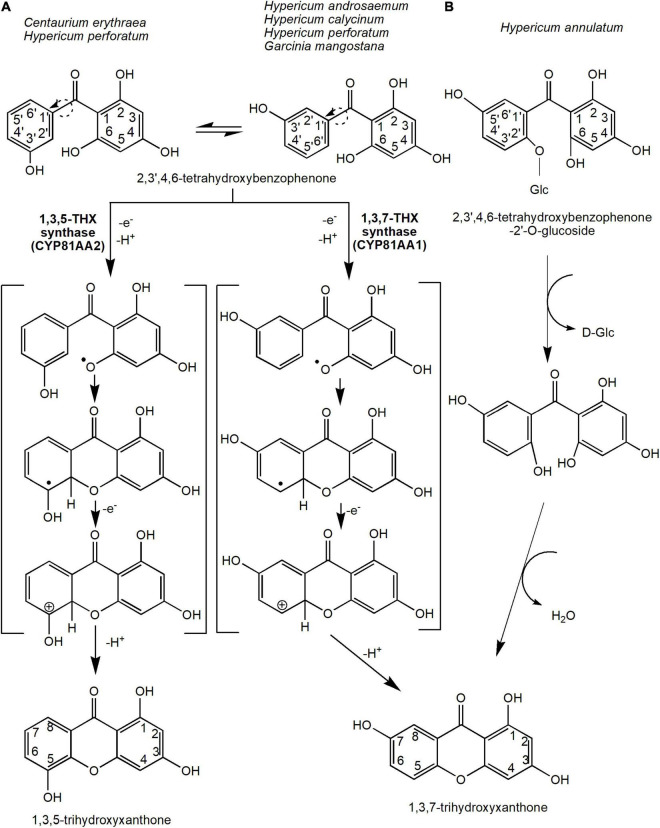
FIGURE 3.
The reaction mechanisms for the two main precursors of xanthones, 1,3,5-trihydroxyxanthone and 1,3,7-trihydroxyxanthone. This oxidative phenol coupling reaction is present in several plant species including C. erythraea, H. androsaemum, H. calycinum, H. perforatum, and G. mangostana. (A) In addition, 1,3,7-trihydroxyxanthone can be formed through the deglycosidation process of 2,3′,4,6-tetrahydroxybenzophenone-2′-O-glucoside in H. annulatum. (B) Dotted arrows indicate hypothetical/proposed pathways while protein activities detected at the molecular level are in bold (refer to Table 1). CYP, cytochrome P450; THX, trihydroxyxanthone.