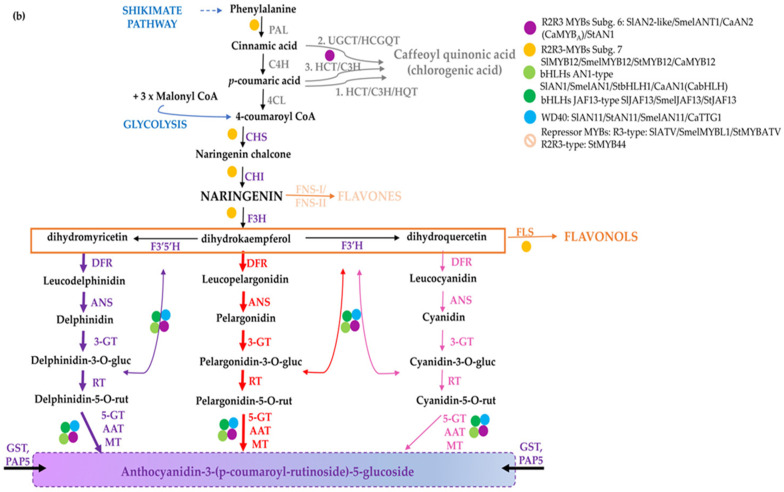
Figure 1.
The flavonoid biosynthesis and its regulation in cereal and Solanaceae crops. Scheme of the pathway leading to the production of flavonoids and phenolic acids in monocots (a) and dicots (b). Phenylalanine is first deaminated by PAL to produce cinnamic acid, then converted by C4H into p-coumaric acid, which can enter the synthesis of hydroxycinnamic acids (i.e., chlorogenic acid and other phenolics) or it can be conjugated with coenzyme A to produce 4-coumaroyl-CoA by 4CL. CHS catalyses the condensation of p-coumaroyl-CoA with three molecules of malonyl-CoA to naringenin chalcone, then converted to the flavanone naringenin by CHI. Indeed, naringenin may be converted to flavones by FNSI/FNSII (e.g., maysin in maize), to the red phlobaphenes, derived from condensation of the 3-deoxy flavonoids apiferol and luteoforol (a) and to dihydroflavonols, such as dihydrokaempferol (DHK), which can then be used by F3′H to produce dihydroquercetin (DHQ) or by F3′5′H to form dihydromyricetin (DHM) (b). Dihydroflavonols are then converted to flavonols (e.g., kaempferol, quercetin, and myricetin) by FLS. Downstream, DFR reduces the dihydroflavonols to their respective colourless leucoanthocyanidins, which are then converted into the coloured anthocyanidins (e.g., cyanidin, pelargonidin, and delphinidin). The main enzymes catalyzing the reactions in the pathway are reported in violet. Regulatory proteins belonging to diverse classes of transcription factors are marked with coloured dots. Branches leading to different classes of flavonoids and anthocyanins are indicated with diverse colours; in B, thicker purple and red arrows highlight the branch leading to the most abundant derived anthocyanins. The name of the enzymes is detailed in the abbreviation list.