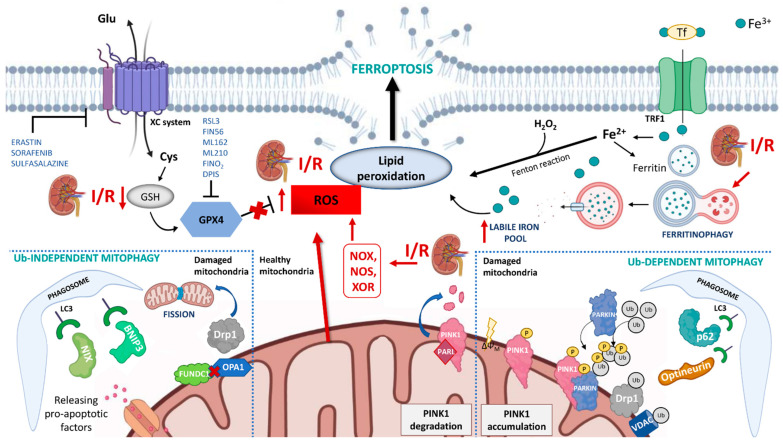
Figure 1.
Schematic representation of the mechanisms of ferroptosis and mitophagy in renal ischemia/reperfusion (I/R) injury. During I/R several pathways contribute to ferroptosis: (i) the overproduction of ROS by NADPH oxidase (NOX), nitric oxide synthase (NOS), xanthine oxidoreductase (XOR) and mitochondria promotes lipid peroxidation and plasmatic membrane rupture; (ii) the reduction in glutathione (GSH) content inhibits glutathione peroxidase 4 (GPX4) activity and its protective action against membrane lipid peroxidation; (iii) I/R can indirectly induce ferritinophagy which causes the degradation of intracellular ferritin, and the increment of intracellular labile iron pool. Mitophagy is activated in I/R through both ubiquitin-dependent and ubiquitin-independent mechanisms and seems to have a protective role in I/R injury by reducing the release of reactive oxygen species from dysfunctional mitochondria. In physiological conditions, PINK1 is imported into mitochondria where it is cleaved by the intramembrane serine protease presenilin associated rhomboid-like (PARL) and ultimately degraded. When mitochondria are damaged, and lose their membrane potential, PINK1 accumulates on the mitochondrial outer membrane (MOM) and recruits Parkin. Parkin ubiquitinates several mitochondrial substrates such as voltage-dependent anion-selective channel protein (VDAC) and dynamin-1-like protein (DRP1). These ubiquitinated proteins can recruit mitophagy receptors (such as optineurin, p62) that link mitochondria to autophagosomes through interacting with LC3. This causes an autophagic engulfment of the organelle necessary for its degradation. The ubiquitin-independent mechanism is regulated by mitophagy receptors that localize on MOM, such as BCL2 interacting protein 3 (BNIP3), BNIP3-like (BNIP3L/NIX), and FUN14 domain containing 1 (FUNDC1). These proteins bridge mitochondria to autophagosome by directly interacting with LC3.