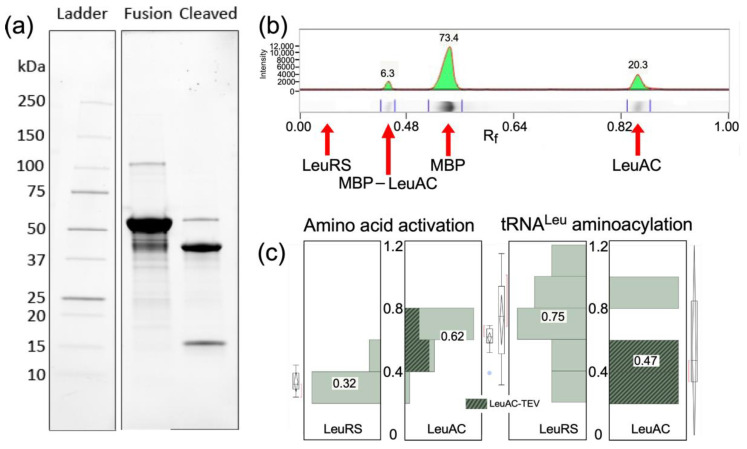
Figure 1.
Purity of LeuAC urzyme. (a) PROTEAN® TGX PAGE gel of purified LeuAC-MBP fusion protein and its TEV-cleaved products. Visualization is proportional to the tryptophan content of each band, as noted in the text. (b) Densitometric scan of the gel in (a). Integrated peak percentages are indicated for full-length LeuRS, LeuAC_MBP fusion, MBP, and LeuAC. (c) Distributions of amplitude parameters, A, estimated by fitting [32P] ATP consumption and tRNALeu aminoacylation time courses for LeuRS and TEV-cleaved LeuAC to Equation (1). Acylation experiments include the subset of experiments from Supplementary Table S2 that most closely approximate single turnover conditions (5 > [substrate]/[enzyme] > 0.5). Median A values given as fractions against a white background are aligned with horizontal lines in the box plots. Median values for activation differ by 11.5 times the standard error of the mean for LeuRS (n = 13). For acylation the difference between median values for full length LeuRS and LeuAC, 0.27, is 3.7 times the standard error of the mean for LeuRS (n = 7). The difference for activation is both larger and inverted because LeuAC converts near stoichiometric amounts of ATP into ADP, in addition to AMP, as discussed in Section 2.3.2 and Section 3.3.