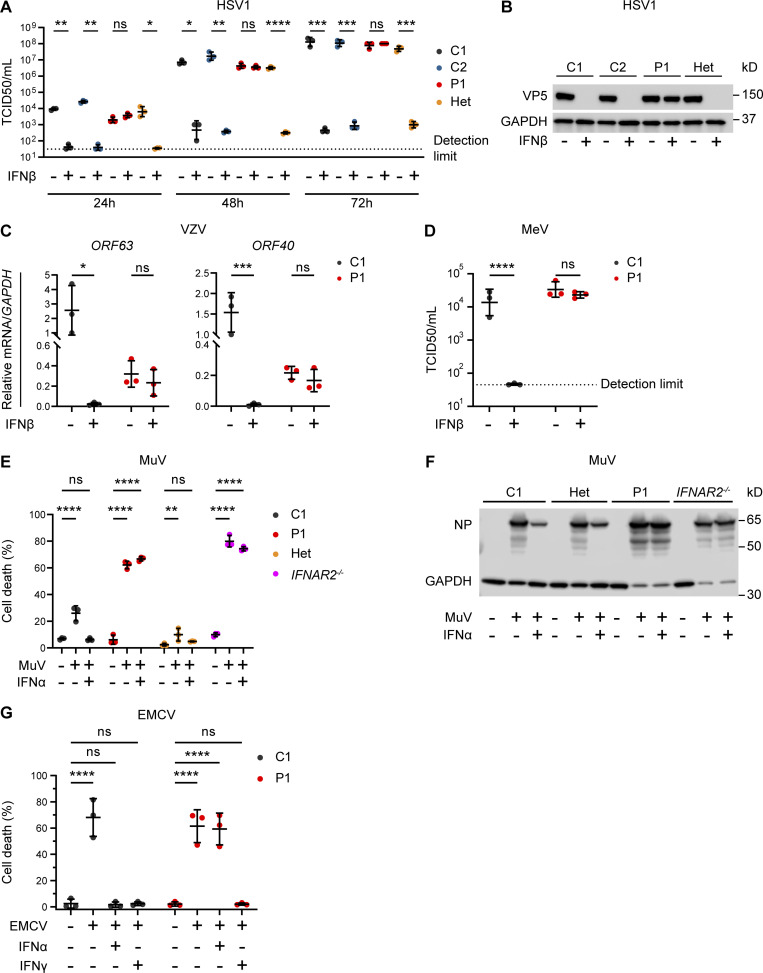
Figure 3.
Impaired viral control in patient fibroblasts bearing homozygous IFNAR2 p.Ser53Pro. (A and B) SV40-immortalized dermal fibroblasts from P1, two healthy controls (C1 and C2), and the heterozygous mother of P1 (Het) were pretreated with IFNβ 100 IU/ml for 24 h before infection with HSV1 (KOS strain) at an MOI of 0.001. (A) At 24, 48, and 72 hpi, supernatants were sampled and titrated for TCID50 (geometric mean ± SD of n = 3 independent repeats; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, two-way ANOVA with Tukey’s test for multiple comparisons). (B) At 72 hpi, the cells were lysed for immunoblotting of whole-cell lysates for the HSV1 protein VP5 and GAPDH as the loading control. One representative immunoblot of n = 3 independent repeat experiments is shown. (C) SV40-immortalized dermal fibroblasts from P1 and a healthy control were pretreated with IFNβ 100 IU/ml for 24 h before infection with cell-free VZV at an MOI of 1. At 48 hpi, total RNA was harvested for RT-qPCR, evaluating the levels of VZV immediate early ORF63 and late ORF40 transcripts, respectively, relative to GAPDH (mean ± SD of n = 3 independent repeats; *, P < 0.05; ***, P < 0.001, two-way ANOVA with Šidák’s test for multiple comparisons). (D) SV40-immortalized dermal fibroblasts from P1 and one healthy control were pretreated with IFNβ 100 IU/ml for 24 h before infection with MeV (Edmonston strain, MOI = 0.00083). At 96 hpi, supernatants were harvested and titrated for TCID50 (geometric mean ± SD of n = 3 independent repeats; ****, P < 0.0001, two-way ANOVA with Tukey’s test for multiple comparisons). (E and F) Primary dermal fibroblasts from P1, a healthy control (C1), the heterozygous mother of P1 (Het) and a known IFNAR2-deficient patient (IFNAR2−/−) were pretreated with IFNα2b 1,000 IU/ml for 16 h before infection with MuV at an MOI 0.1 (MuV, Enders strain). At 72 hpi, (E) viability was assessed in an imaging based live cell viability assay (mean ± SD of n = 3 independent repeats; **, P < 0.01; ****, P < 0.0001, two-way ANOVA with Dunnett’s test for multiple comparisons) and (F) whole cell lysates were prepared for immunoblotting and visualization of MuV nucleoprotein (NP) and GAPDH as loading control. One representative immunoblot of n = 3 independent repeat experiments is shown. (G) Primary dermal fibroblasts from P1 and a healthy control (C1) were pretreated with IFNα2b 1,000 IU/ml for 16 h before infection with a cytopathic dose of EMCV. At 24 hpi, cell viability was assessed in an imaging based live cell viability assay (mean ± SD of n = 3 independent repeats; ****, P < 0.0001, two-way ANOVA with Dunnett’s test for multiple comparisons). Source data are available for this figure: SourceData F3.