Scheme 2. (A) Synthesis of tert-Butyl Ester and Amide Derivatives. (B) Synthesis of Boc-Protected Aniline, Acetamide, and Sulfonamide Derivatives.
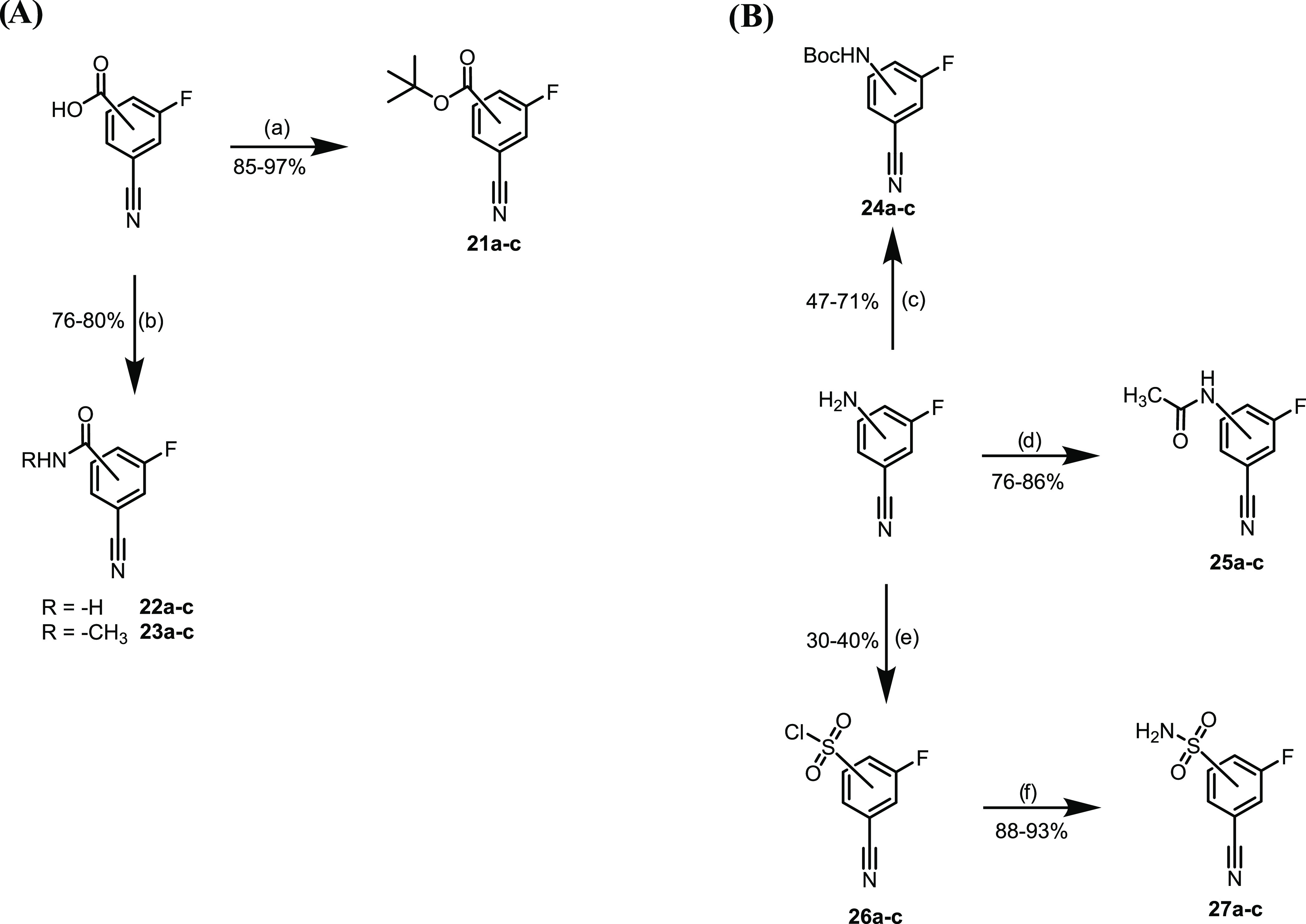
(a) tBuOH, 4-dimethylaminopyridine (DMAP), Boc2O, tetrahydrofuran (THF), rt, and 12 h; (b) NH3 (35% in H2O) or CH3NH2 (40% in H2O), CDI, CH3CN, rt, and 2 h; (c) Boc2O, Et3N, CH2Cl2, rt, and 12 h; (d) Ac2O, CH2Cl2, rt, and 24 h; (e) (i) HCl, NaNO2, 0 °C to rt, and 2 h; (ii) SO2, CuCl, AcOH, 0 °C to rt, and 1 h; and (f) NH3, MeOH, CH3CN, 0 °C to rt, and 2 h.
