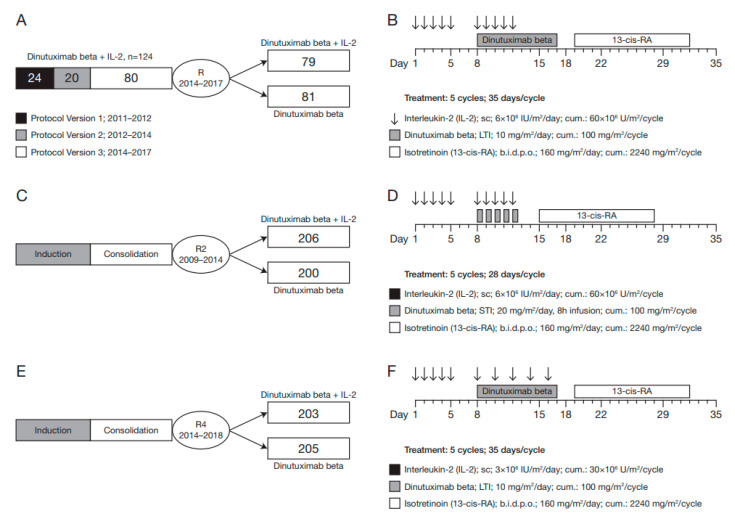
Figure 1.
Schematic overview of study designs and treatment schedules in the LTI/SIOPEN study and the HR-NBL1/SIOPEN study. (A,B) Study design and treatment schedule of the LTI study. The study was initiated as a single-arm study of DB combined with scIL-2 in patients with relapsed/refractory high-risk neuroblastoma and amended in 2014 to include a randomizat design. Patients received either DB alone or DB combined with scIL-2. DB was administered as LTI (10 mg/m2 continuous infusion over 10 days; total dose 100 mg/m2). (C,D) Study design and treatment schedule of HR-NBL1-R2. Newly diagnosed patients with high-risk neuroblastoma were randomizat in the maintenance treatment phase to receive either DB alone or DB combined with scIL-2. DB was administered as STI (20 mg/m2/day on 5 consecutive days, 8 h infusions; total dose 100 mg/m2). (E,F) Study design and treatment schedule of HR-NBL1-R4. The study was amended to evaluate LTI of DB and a dose-reduced relaxed schedule of scIL-2. B.i.d.p.o., twice-daily oral administration; DB, dinutuximab beta; LTI, long-term infusion; R, randomization; RA, retinoic acid; scIL-2, subcutaneous interleukin-2; STI, short-term infusion.